258040
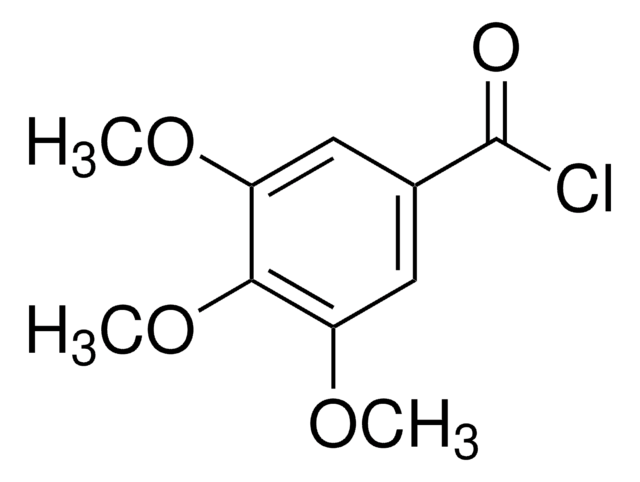
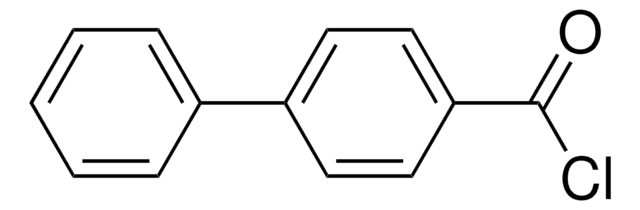
3,4-Dimethoxybenzoyl chloride
98%
Synonym(s):
Veratroyl chloride
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(CH3O)2C6H3COCl
CAS Number:
Molecular Weight:
200.62
Beilstein:
783596
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
70-73 °C (lit.)
functional group
acyl chloride
SMILES string
COc1ccc(cc1OC)C(Cl)=O
InChI
1S/C9H9ClO3/c1-12-7-4-3-6(9(10)11)5-8(7)13-2/h3-5H,1-2H3
InChI key
VIOBGCWEHLRBEP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
3,4-Dimethoxybenzoyl chloride has been used in synthesis of:
- (+)-5-(3,4-dimethoxyphenyl)-4-[[N-[(4S)-2-oxo-4-(phenylmethyl)-2-oxazolidinyl]]carbonyl] oxazole and its enantiomer
- N-(2,5-dibromophenyl)-3,4-dimethoxybenzamide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Jian S. Tang et al.
The Journal of organic chemistry, 61(25), 8750-8754 (1996-12-13)
Both (4S-(+)-3-(isocyanoacetyl)-4-(phenylmethyl)-2-oxazolidinone (R)-1 and its enantiomer (S)-1 have been synthesized as potentially useful synthons in asymmetric synthesis. Optically active (+)-5-(3,4-dimethoxyphenyl)-4-[[N-[(4S)-2-oxo-4-(phenylmethyl)-2-oxazolidinyl]]carbonyl] oxazole (S)-2 and its enantiomer (R)-2 obtained by treating 3,4-dimethoxybenzoyl chloride with (S)-1 and (R)-1, respectively, in the presence of
Catriona G Mortimer et al.
Journal of medicinal chemistry, 49(1), 179-185 (2006-01-06)
A series of new 2-phenylbenzothiazoles has been synthesized on the basis of the discovery of the potent and selective in vitro antitumor properties of 2-(3,4-dimethoxyphenyl)-5-fluorobenzothiazole (8n; GW 610, NSC 721648). Synthesis of analogues substituted in the benzothiazole ring was achieved
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service