235296
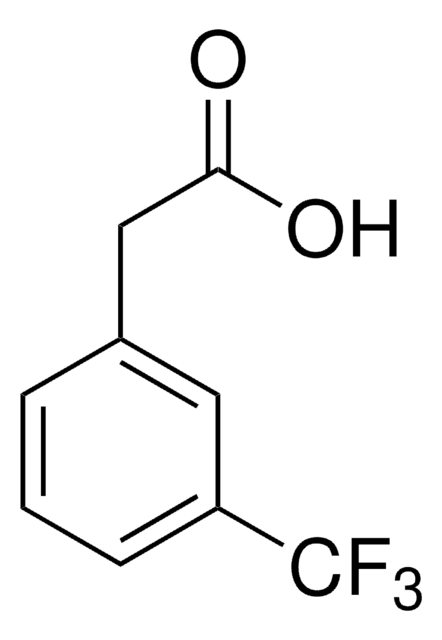
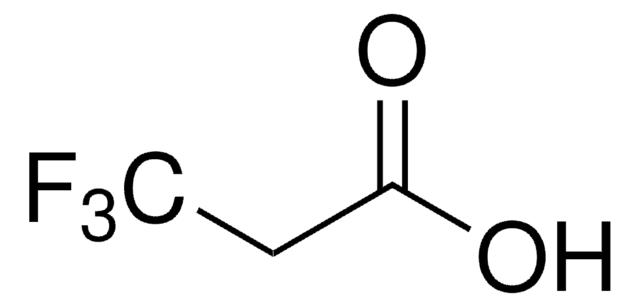
2-(Trifluoromethyl)phenylacetic acid
98%
Synonym(s):
(α,α,α-Trifluoro-o-tolyl)acetic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CF3C6H4CH2CO2H
CAS Number:
Molecular Weight:
204.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
100-102 °C (lit.)
functional group
carboxylic acid
fluoro
SMILES string
OC(=O)Cc1ccccc1C(F)(F)F
InChI
1S/C9H7F3O2/c10-9(11,12)7-4-2-1-3-6(7)5-8(13)14/h1-4H,5H2,(H,13,14)
InChI key
TYOCDHCKTWANIR-UHFFFAOYSA-N
Application
2-(Trifluoromethyl)phenylacetic acid has been used in the synthesis of potential antithrombotics and lipoxygenase inhibitors.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
P L Barker et al.
Journal of medicinal chemistry, 35(11), 2040-2048 (1992-05-29)
Stimulation of platelets activates GPIIbIIIa, the heterodimeric integrin receptor, to bind fibrinogen (Fg), which results in platelet aggregation. GPIIbIIIa/Fg binding inhibitors are potentially suitable for acute use during and after thrombolytic therapy as antithrombotic agents. Incorporation of the tripeptide sequence
E S Lazer et al.
Journal of medicinal chemistry, 33(7), 1892-1898 (1990-07-01)
A series of 2,6-disubstituted 4-(2-arylethenyl)phenols with potent human neutrophil 5-lipoxygenase (5-LO) inhibiting activity (IC50S in the 10(-7) M range) and weaker human platelet cyclooxygenase (CO) inhibiting activity (IC50S in the 10(-6) M range) is described. This series evolved from the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service