226998
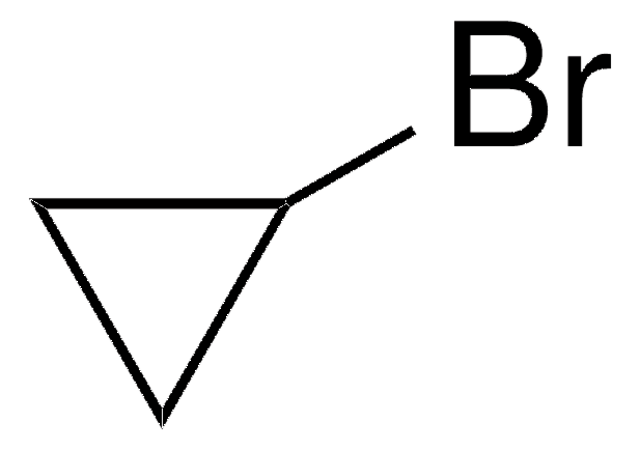
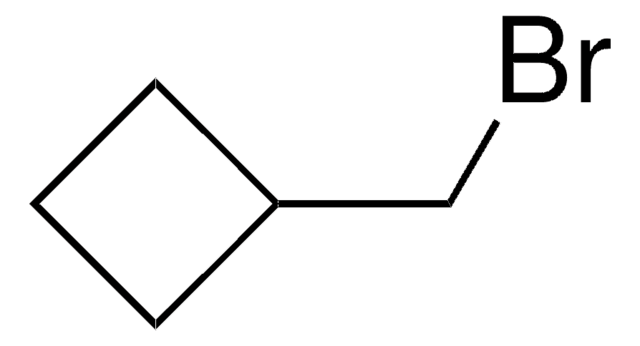
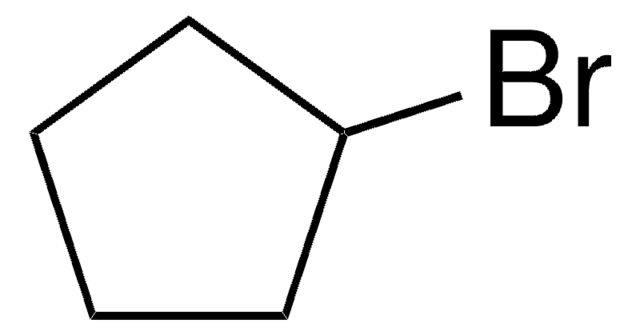
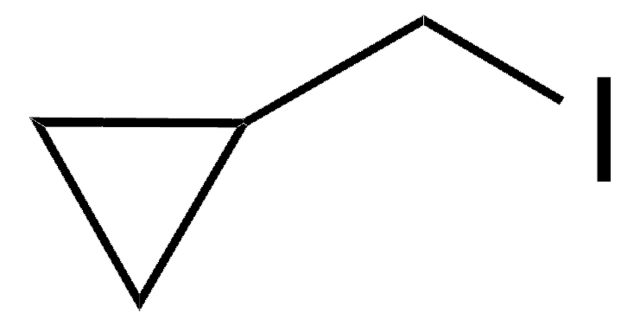
Bromocyclobutane
96%
Synonym(s):
Cyclobutyl bromide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H7Br
CAS Number:
Molecular Weight:
135.00
Beilstein:
1900415
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
form
liquid
refractive index
n20/D 1.479 (lit.)
bp
108 °C (lit.)
density
1.434 g/mL at 25 °C (lit.)
functional group
bromo
SMILES string
BrC1CCC1
InChI
1S/C4H7Br/c5-4-2-1-3-4/h4H,1-3H2
InChI key
KXVUSQIDCZRUKF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
accessory
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
71.6 °F - closed cup
Flash Point(C)
22 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yi Liu et al.
The journal of physical chemistry. A, 110(16), 5379-5385 (2006-04-21)
This study investigates the 234 nm photodissociation dynamics of cyclobutyl bromide using a two-dimensional photofragment velocity imaging technique. The spin-orbit ground- and excited-state Br(2P) atoms are state-selectively detected via [2+1] resonance enhanced multiphoton ionization (REMPI), whereas the cyclobutyl radicals are
Zhen Li et al.
Journal of medicinal chemistry, 48(20), 6169-6173 (2005-09-30)
A class of 3,5-diphenyl-1,2,4-oxadiazole based compounds have been identified as potent sphingosine-1-phosphate-1 (S1P1) receptor agonists with minimal affinity for the S1P2 and S1P3 receptor subtypes. Analogue 26 (S1P1 IC50 = 0.6 nM) has an excellent pharmacokinetics profile in the rat
Tao Guo et al.
Bioorganic & medicinal chemistry letters, 15(16), 3696-3700 (2005-06-28)
An encoded combinatorial library based on aryl and biaryl piperidine scaffolds was designed and synthesized. Screening of this library resulted in the discovery of high-nanomolar biaryl piperidine-based MCH1 receptor antagonists. Follow-up optimization using a parallel synthesis provided potent, single digit
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service