All Photos(1)
About This Item
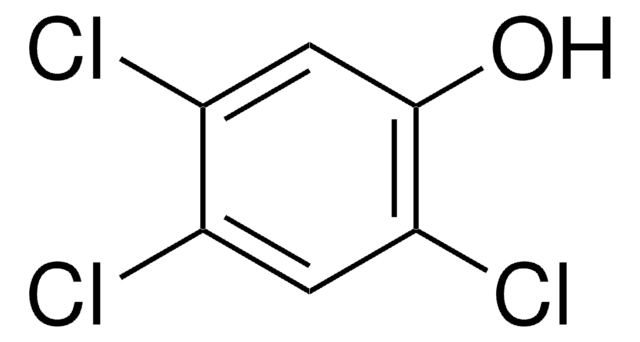
Empirical Formula (Hill Notation):
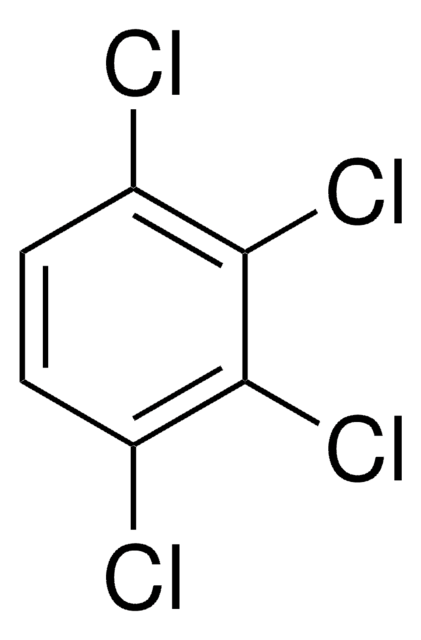
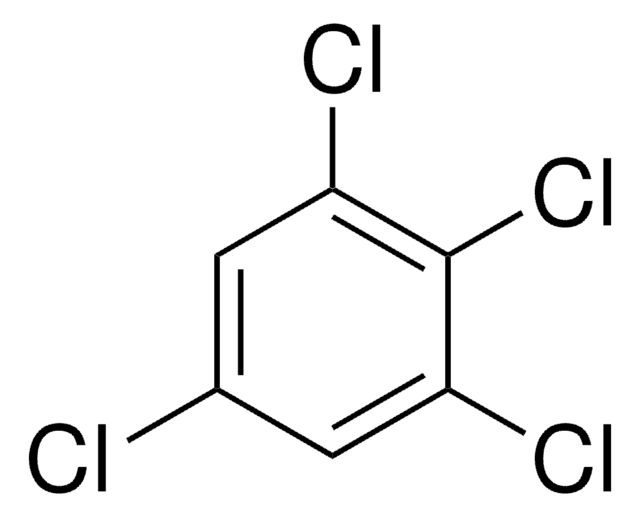
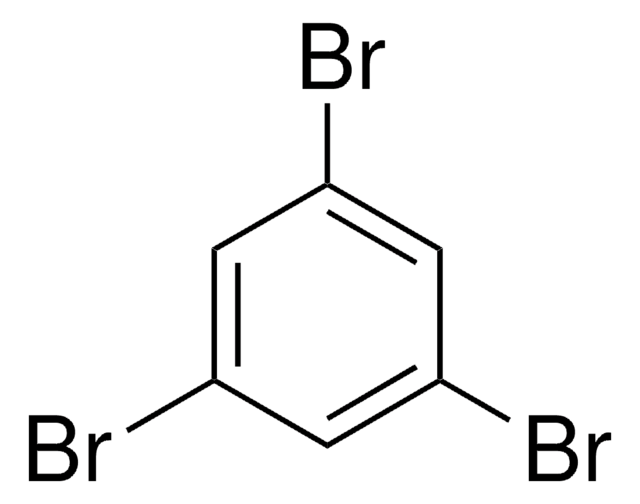
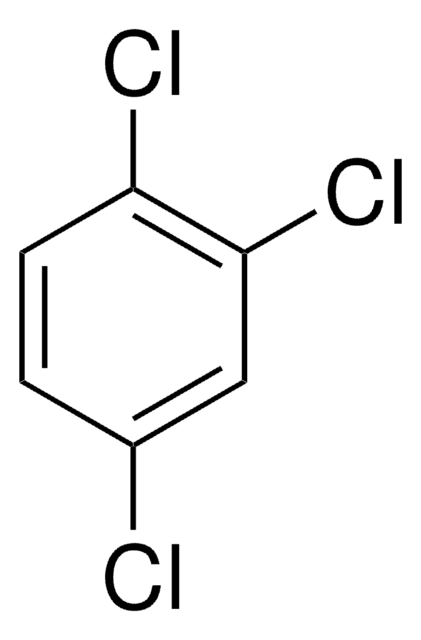
C6H2Cl4
CAS Number:
Molecular Weight:
215.89
Beilstein:
1618315
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
chips
bp
240-246 °C (lit.)
mp
138-140 °C (lit.)
SMILES string
Clc1cc(Cl)c(Cl)cc1Cl
InChI
1S/C6H2Cl4/c7-3-1-4(8)6(10)2-5(3)9/h1-2H
InChI key
JHBKHLUZVFWLAG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
1,2,4,5-Tetrachlorobenzene was used to study the environmental behavior of hexachlorobutadiene (HCBD).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
320.0 °F - closed cup
Flash Point(C)
160 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Haiyan Zhang et al.
Environmental science & technology, 48(3), 1525-1531 (2014-01-10)
Although hexachlorobutadiene (HCBD) was recently proposed as a candidate persistent organic pollutant (POP) under the Stockholm Convention, information about its environmental levels and distributions is still very limited. In this work, HCBD was determined in the sewage sludge from 37
Lin Xiao et al.
Environmental science & technology, 41(8), 2750-2755 (2007-05-31)
We observed that the presence of transition metal ion, Ag+, Cu2+, or Fe3+, at a concentration of 3 mg L(-1) increases sorption of two nonpolar hydrophobic organic compounds (HOCs), phenanthrene (PHEN), and 1,2,4,5-tetrachlorobenzene (TeCB) by 1.5-4 times to Gram-negative bacteria
Motoki Terashima et al.
Chemosphere, 57(6), 439-445 (2004-09-08)
Solubilizing abilities of aggregates of humic acid (HA) to chlorinated benzenes (CBs) were investigated by means of the apparent water solubility enhancement. Both the water solubilities of 1,4-dichlorobenzene (DCB) and 1,2,4,5-tetrachlorobenzene (TeCB) linearly increased with increasing concentration of HA above
S Beil et al.
Journal of bacteriology, 180(21), 5520-5528 (1998-10-29)
The TecA chlorobenzene dioxygenase and the TodCBA toluene dioxygenase exhibit substantial sequence similarity yet have different substrate specificities. Escherichia coli cells producing recombinant TecA enzyme dioxygenate and simultaneously eliminate a halogen substituent from 1,2,4,5-tetrachlorobenzene but show no activity toward benzene
Oxygen consumption of juvenile rainbow trout (Oncorhynchus mykiss) exposed to sublethal concentrations of 1,2,4,5-tetrachlorobenzene and tetrachloroguaiacol.
R Yang et al.
Bulletin of environmental contamination and toxicology, 59(3), 479-485 (1997-09-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service