1A01040
USP
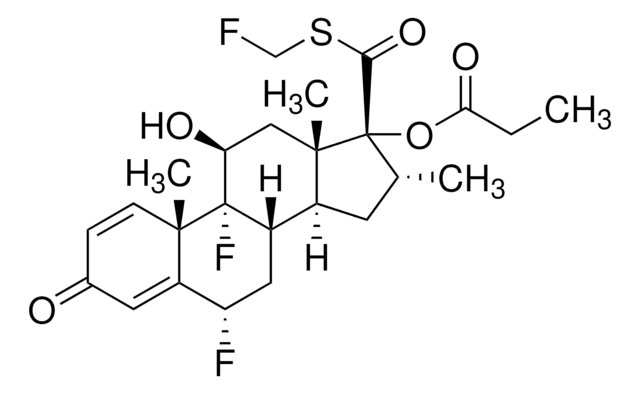
11-Ketofluticasone Propionate
Pharmaceutical Analytical Impurity (PAI)
Synonym(e):
6alpha, 9-difluoro-17- [[(fluoromethyl)sulfanyl] carbonyl]-16alpha-methyl-3,11-dioxoandrosta-1-4dien-17alpha-yl-propanoate, (6S,8S,9R,10S,13S,14S,16R,17R)-6,9-Difluoro-17-(((fluoromethyl)thio) carbonyl)-10,13,16-trimethyl-3,11-dioxo-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-17-yl propionate, (6S,8S,9R,10S,13S,14S,16R,17R)-6,9-Difluoro-17-(((fluoromethyl)thio)carbonyl)-10,13,16-trimethyl-3,11-dioxo6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-17-yl propionate
About This Item
Empfohlene Produkte
Qualität
pharmaceutical analytical impurity (PAI)
Agentur
USP
API-Familie
fluticasone
Hersteller/Markenname
USP
Anwendung(en)
pharmaceutical
Format
neat
Lagertemp.
2-8°C
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
USP PAI are a product line of impurities suitable for research and analytical purposes, which help to ensure the quality and safety of medicines.
Associated Drug Substance: Fluticasone Propionate
Therapeutic Area: Steroids
For more information about this PAI, visit here.
Anwendung
Leistungsmerkmale und Vorteile
1. Conduct analytical tests during early formulation feasibility studies.
2. Determine degradation impurities produced during stress studies.
3. Develop, validate, and transfer analytical methods.
4. Perform spiking studies during process R&D to demonstrate depletion upon recrystallization.
5. Record retention times and/or spectra
6. Determine relative response factors.
7. Identify unknown impurities that formed during ICH stability conditions.
8. Identify impurities that are present in the Reference Listed Drug
9. Test for and profile impurities not listed in drug substance and drug product monographs.
Hinweis zur Analyse
Sonstige Hinweise
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.