Wichtige Dokumente
T6137
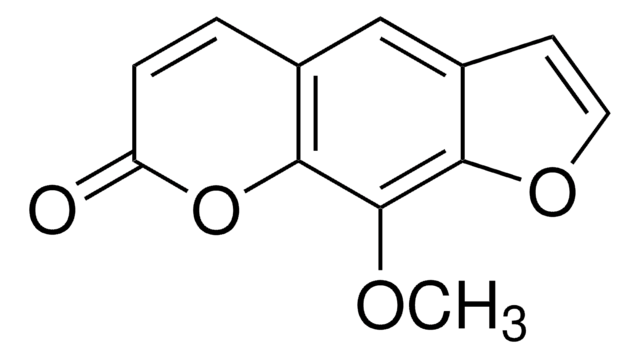
Trioxsalen
≥98% (HPLC), powder, photochemical DNA crosslinker
Synonym(e):
2,5,9-Trimethylfuro[3,2-g]benzopyran-7-on, 4,5′,8-Trimethyl-psoralen, TMP, Trisoralen
About This Item
Empfohlene Produkte
Produktbezeichnung
Trioxsalen, ≥98% (HPLC), powder
Qualitätsniveau
Assay
≥98% (HPLC)
Form
powder
Farbe
white
mp (Schmelzpunkt)
229-231 °C (lit.)
Löslichkeit
chloroform: soluble 50 mg/mL, clear, colorless to faintly yellow
Fluoreszenz
λex 269 nm; λem 445 nm in methanol
λex 321 nm; λem 445 nm (bound to DNA in Tris, pH 8.1)
Ersteller
Valeant
Lagertemp.
−20°C
SMILES String
Cc1cc2cc3C(C)=CC(=O)Oc3c(C)c2o1
InChI
1S/C14H12O3/c1-7-4-12(15)17-14-9(3)13-10(6-11(7)14)5-8(2)16-13/h4-6H,1-3H3
InChIKey
FMHHVULEAZTJMA-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Anwendung
- to induce small deletion mutations in worms
- in combination with ultraviolet A (UVA)
- to induce interstrand crosslinks (ICLs) in DNA
- for the preparation and photoactivation of trimethyl psoralen
Biochem./physiol. Wirkung
Leistungsmerkmale und Vorteile
Angaben zur Herstellung
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Eye Dam. 1 - Muta. 2 - Skin Corr. 1B
Lagerklassenschlüssel
8A - Combustible corrosive hazardous materials
WGK
WGK 2
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
DNA damage and repair mechanism is vital for maintaining DNA integrity. Damage to cellular DNA is involved in mutagenesis, the development of cancer among others.
Verwandter Inhalt
n proliferating cells, the cell cycle consists of four phases. Gap 1 (G1) is the interval between mitosis and DNA replication that is characterized by cell growth. Replication of DNA occurs during the synthesis (S) phase, which is followed by a second gap phase (G2) during which growth and preparation for cell division occurs. Together, these three stages comprise the interphase phase of the cell cycle. Interphase is followed by the mitotic (M) phase.
Apoptosis, or programmed cell death (PCD), is a selective process for the removal of unnecessary, infected or transformed cells in various biological systems. As it plays a role in the homeostasis of multicellular organisms, apoptosis is tightly regulated through two principal pathways by a number of regulatory and effector molecules.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.









![2-Amino-9H-pyrido[2-3-b]indole ≥98% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/351/152/37c98523-590f-4b27-826c-5b3d4b502047/640/37c98523-590f-4b27-826c-5b3d4b502047.png)