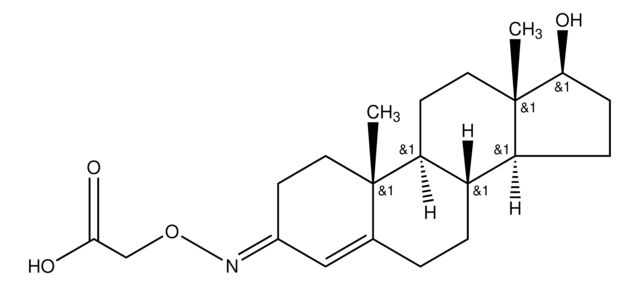
T5771
Testosterone 3-(O-carboxymethyl)oxime: BSA - fluorescein isothiocyanate conjugate
About This Item
Empfohlene Produkte
Assay
≥98.00% (TLC)
Qualitätsniveau
Form
powder
Arzneimittelkontrolle
regulated under CDSA - not available from Sigma-Aldrich Canada
Kennzeichnungsgrad
~3 mol FITC per mol BSA
~10 mol steroid per mol BSA
Methode(n)
flow cytometry: suitable
Löslichkeit
water: 1.90-2.10 mg/mL, clear to faintly hazy, orange
Versandbedingung
ambient
Lagertemp.
2-8°C
Anwendung
Biochem./physiol. Wirkung
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Carc. 2
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.