Wichtige Dokumente
SAB4200711
Anti-Human Serum Albumin antibody, Mouse monoclonal
clone HSA-11, purified from hybridoma cell culture
Synonym(e):
ALB
About This Item
Empfohlene Produkte
Biologische Quelle
mouse
Qualitätsniveau
Antikörperform
purified from hybridoma cell culture
Antikörper-Produkttyp
primary antibodies
Klon
HSA-11, monoclonal
Form
buffered aqueous solution
Mol-Gew.
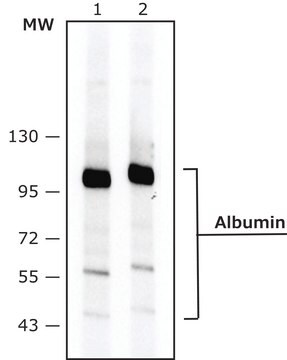
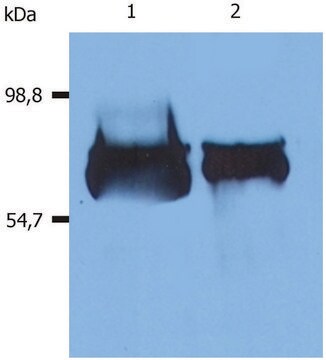
~70 kDa
Speziesreaktivität
gibbon, monkey, baboon
Konzentration
~1.0 mg/mL
Methode(n)
immunoblotting: 2.5-5 ng/mL using human serum
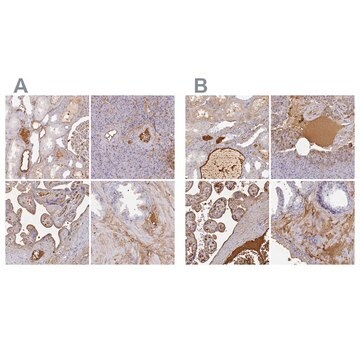
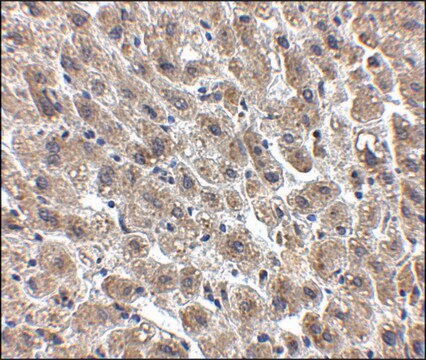
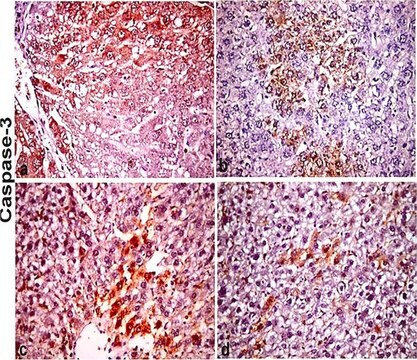
immunohistochemistry: suitable
indirect ELISA: 0.2-0.4 μg/mL using 10 μg/ml Human Serum Albumin for coating
Isotyp
IgG2a
UniProt-Hinterlegungsnummer
Versandbedingung
dry ice
Lagertemp.
−20°C
Posttranslationale Modifikation Target
unmodified
Angaben zum Gen
human ... ALB(213)
Allgemeine Beschreibung
HSA is encoded by the gene mapped to human chromosome 4q13.3. It is characterized with three homologous domains that assemble to form a heart-shaped molecule. HSA constitutes around 60-65% of total serum protein.
The human serum albumin (HSA) gene is located on human chromosome 4. It encodes a globular protein that is the most prominent protein in human sera. It reaches a total concentration of about 60% of the total proteins in the blood serum. The globular protein is composed of 585 amino acids and comprises of three globular domains that resemble each other in structure, each containing two subdomains.
Spezifität
Immunogen
Anwendung
Biochem./physiol. Wirkung
Human serum albumin (HSA) is a single non-glycosylated chain that has excellent binding capacity for various endogenous and exogenous ligands. It functions as a plasma transporter molecule and primarily binds to non-esterified long-chain fatty acids. It also binds to and transports various metabolites, such as bilirubin, steroid hormones, thyroxine, tryptophan, certain vitamins and metal ions within the body. It also has the ability to bind to several drugs and affects their pharmacokinetics and pharmacodynamics. HSA functions as a NO-carrier and is also responsible for the antioxidant capacity of human serum. In cases of acute hemolysis, HSA binds to heme in the blood stream and transports to hemopexin, where in it is reabsorbed by parenchymal liver cells.
Physikalische Form
Lagerung und Haltbarkeit
Haftungsausschluss
Sie haben nicht das passende Produkt gefunden?
Probieren Sie unser Produkt-Auswahlhilfe. aus.
Lagerklassenschlüssel
10 - Combustible liquids
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
Die passende Version wird nicht angezeigt?
Wenn Sie eine bestimmte Version benötigen, können Sie anhand der Lot- oder Chargennummer nach einem spezifischen Zertifikat suchen.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.