Alle Fotos(1)
Wichtige Dokumente
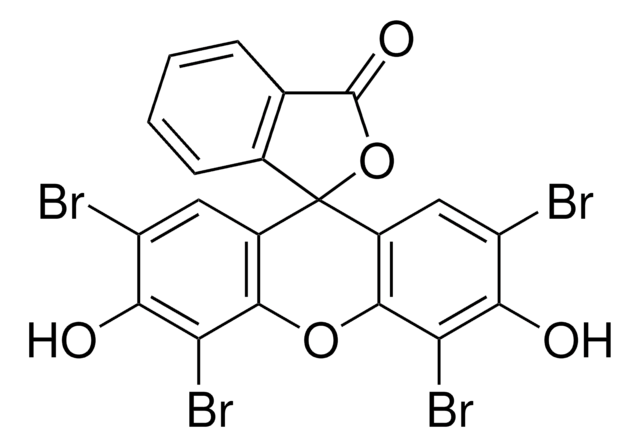
G3416
5′-Guanidinonaltrindole di(trifluoroacetate) salt hydrate
solid, ≥98% (HPLC)
Synonym(e):
GNTI
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C27H29N5O3 · 2C2HF3O2 · xH2O
CAS-Nummer:
Molekulargewicht:
699.60 (anhydrous basis)
MDL-Nummer:
UNSPSC-Code:
12352202
PubChem Substanz-ID:
NACRES:
NA.77
Empfohlene Produkte
Assay
≥98% (HPLC)
Form
solid
Löslichkeit
H2O: 30 mg/mL
Lagertemp.
2-8°C
Angaben zum Gen
human ... OPRK1(4986)
Anwendung
5′-Guanidinonaltrindole di(trifluoroacetate) salt hydrate (GNTI) has been used as a selective κ opioid receptor antagonist:
- to study the influence of opioid receptor types on the anti-hyperalgesic effect of dipeptidyl peptidase 4 (DPP4) inhibitors in inflammation
- to study the role of κ opioid receptor in the forebrain-dependent associative task, Whisker-Trace Eyeblink conditioning (WTEB)
- to validate its inhibitory actions on Akt kinase activities
Biochem./physiol. Wirkung
5′-Guanidinonaltrindole di(trifluoroacetate) (GNTI) is a 5‘-guanidine derivative and a selective κ opiate receptor antagonist. It is five-fold more potent and 500-fold more selective than norbinaltorphimine (nor-BNI) for the κ opioid receptor in smooth muscle preparations.
Leistungsmerkmale und Vorteile
This compound is featured on the Opioid Receptors page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
Rechtliche Hinweise
Sold under US Patent No. 6,500,824.
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
Lot/Batch Number
Die passende Version wird nicht angezeigt?
Wenn Sie eine bestimmte Version benötigen, können Sie anhand der Lot- oder Chargennummer nach einem spezifischen Zertifikat suchen.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
W C Stevens et al.
Journal of medicinal chemistry, 43(14), 2759-2769 (2000-07-14)
The indole moiety in the delta-opioid antagonist, naltrindole (2, NTI), was employed as a scaffold to hold an "address" for interaction with the kappa-opioid receptor. The attachment of the address to the 5'-position of the indole moiety was based on
Pathology and glia type specific changes of the DPP4 activity in the spinal cord contributes to the development and maintenance of hyperalgesia and shapes opioid signalling in chronic pain states
KiralyK, et al.
Scientific reports (2017)
R M Jones et al.
European journal of pharmacology, 396(1), 49-52 (2000-05-24)
5'-Guanidinonaltrindole (GNTI) possesses 5-fold greater opioid antagonist potency (K(e)=0.04 nM) and an order of magnitude greater selectivity (selectivity ratios >500) than the prototypical kappa-opioid receptor antagonist, norbinaltorphimine, in smooth muscle preparations. Binding and functional studies conducted on cloned human opioid
Pathology and glia type specific changes of the DPP4 activity in the spinal cord contributes to the development and maintenance of hyperalgesia and shapes opioid signalling in chronic pain states
KiralyK, et al.
Scientific Reports (2017)
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.