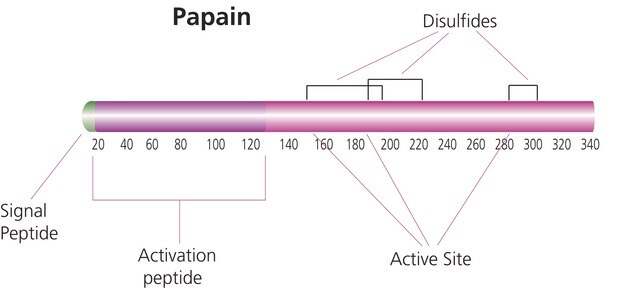
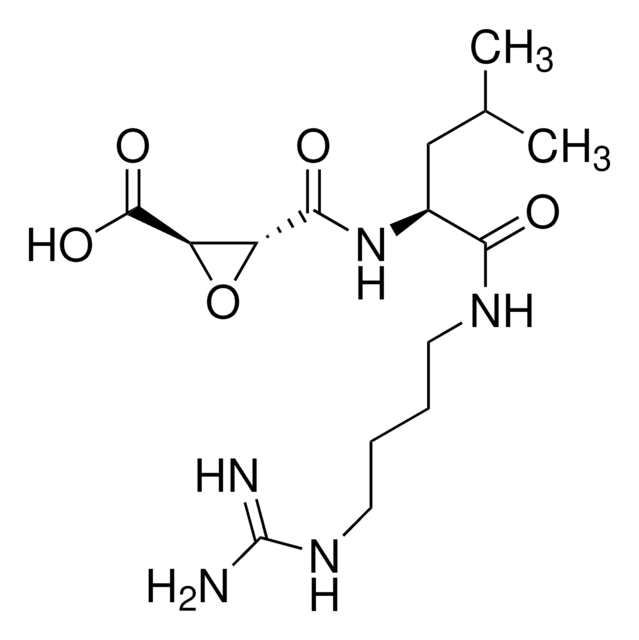
F4165
Ficin from fig tree latex
powder, ≥0.1 unit/mg solid
Synonym(e):
Debricin, Ficain, higueroxyl delabarre
About This Item
Empfohlene Produkte
Biologische Quelle
fig tree (latex)
Qualitätsniveau
Form
powder
Spezifische Aktivität
≥0.1 unit/mg solid
Mol-Gew.
23.8 kDa
Lagertemp.
−20°C
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
pI: 9.0
Biochem./physiol. Wirkung
Einheitendefinition
Inhibitor
Substrat
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 1
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Protokolle
This procedure may be used for all Ficin products.
This procedure may be used for all Ficin products.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.