Wichtige Dokumente
E6910
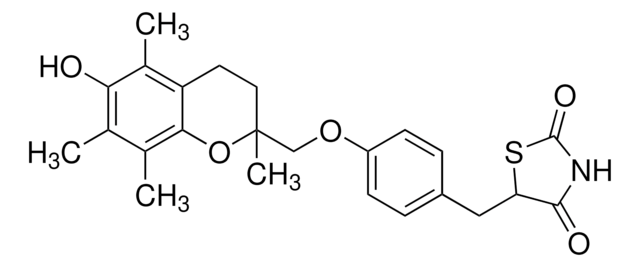
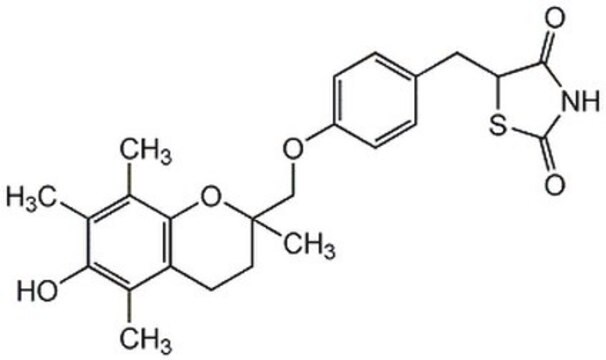
Pioglitazon -hydrochlorid
≥98% (HPLC), powder, hepatic gluconeogenesis blocker
Synonym(e):
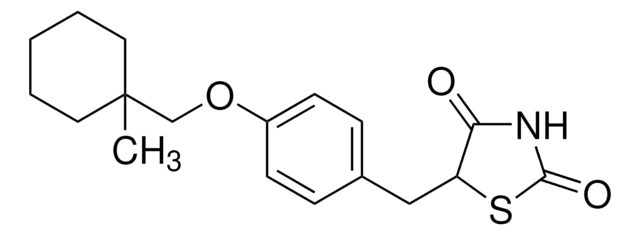
5-[[4-[2-(5-Ethyl-2-pyridinyl)-ethoxy]-phenyl]-methyl]-2,4-thiazolidindion -monohydrochlorid
About This Item
Empfohlene Produkte
Produktbezeichnung
Pioglitazon -hydrochlorid, ≥98% (HPLC)
Qualitätsniveau
Assay
≥98% (HPLC)
Form
powder
Farbe
white to off-white
Löslichkeit
DMSO: ≥10 mg/mL
Ersteller
Takeda
Lagertemp.
room temp
SMILES String
Cl.CCc1ccc(CCOc2ccc(CC3SC(=O)NC3=O)cc2)nc1
InChI
1S/C19H20N2O3S.ClH/c1-2-13-3-6-15(20-12-13)9-10-24-16-7-4-14(5-8-16)11-17-18(22)21-19(23)25-17;/h3-8,12,17H,2,9-11H2,1H3,(H,21,22,23);1H
InChIKey
GHUUBYQTCDQWRA-UHFFFAOYSA-N
Angaben zum Gen
human ... PPARG(5468)
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
- to administer to mice model and treated the hepatoma cell line to study its effect on regulating insulin-degrading enzyme (IDE) in diet-induced obese (DIO) C57BL/6 mice
- in drug preparation to analyze its effects on shortening and calcium transport in ventricular myocytes from the Goto-Kakizaki (GK) type 2 diabetic rat
- to treat HepG2 cells with peroxisome proliferator-activated receptor γ (PPARγ) agonists to examine its effect on TOMM40-, APOE- and APOC1-mRNA levels
Biochem./physiol. Wirkung
Leistungsmerkmale und Vorteile
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Oral - Carc. 2
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Verwandter Inhalt
Discover Bioactive Small Molecules for ADME/Tox
Discover Bioactive Small Molecules for Lipid Signaling Research
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.