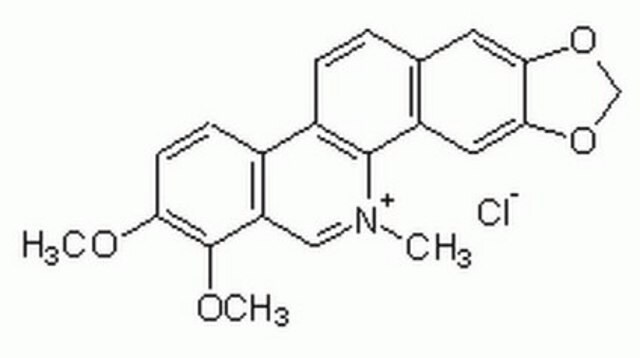
C2932
Chelerythrinchlorid
≥95% (TLC), powder
Synonym(e):
Toddalinchlorid
About This Item
Empfohlene Produkte
Biologische Quelle
plant
Qualitätsniveau
Assay
≥95% (TLC)
Form
powder
Farbe
yellow to orange
mp (Schmelzpunkt)
213.0-214.0 °C
Löslichkeit
DMSO: 2 mg/mL
Lagertemp.
−20°C
SMILES String
Cl.COc1ccc2-c3ccc4cc5OCOc5cc4c3[N](C)=Cc2c1OC
InChI
1S/C21H18NO4.ClH/c1-22-10-16-13(6-7-17(23-2)21(16)24-3)14-5-4-12-8-18-19(26-11-25-18)9-15(12)20(14)22;/h4-10H,11H2,1-3H3;1H
InChIKey
SUPBMPBJXZDANZ-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
- as a supplement in heat-inactivated late-embryo extract or late-embryo extract to inhibit protein kinase C (PKC) activity
- for in vitro Xenopus experiments
- as a PKC inhibitor in HL-1 cells, to block the PKC pathway to study its effects on doxazosin-induced galectin-3 and collagen expression
Biochem./physiol. Wirkung
Leistungsmerkmale und Vorteile
Signalwort
Warning
Gefahreneinstufungen
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.