Wichtige Dokumente
A143
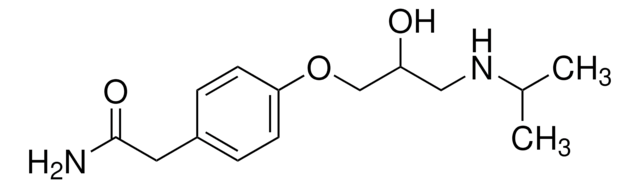
(S)-(−)-Atenolol
powder
Synonym(e):
(−)-4-[2-Hydroxy-3-[(1-methylethyl)amino]propoxy]Benzeneacetamid
About This Item
Empfohlene Produkte
Form
powder
Qualitätsniveau
Optische Aktivität
[α]23/D −10.5°, c = 0.5 in H2O(lit.)
Farbe
white to beige
mp (Schmelzpunkt)
148-152 °C (lit.)
Löslichkeit
0.1 M HCl: soluble
SMILES String
CC(C)NC[C@H](O)COc1ccc(CC(N)=O)cc1
InChI
1S/C14H22N2O3/c1-10(2)16-8-12(17)9-19-13-5-3-11(4-6-13)7-14(15)18/h3-6,10,12,16-17H,7-9H2,1-2H3,(H2,15,18)/t12-/m0/s1
InChIKey
METKIMKYRPQLGS-LBPRGKRZSA-N
Angaben zum Gen
human ... ADRB1(153)
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Anwendung
Biochem./physiol. Wirkung
Anwendung
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Artikel
Our new product range of pharmaceutical impurity solutions as certified reference materials (CRMs) save costs and labor by providing all impurities listed in the USP as well as in the EP in one mix.
Chromatograms
application for HPLCUnser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.