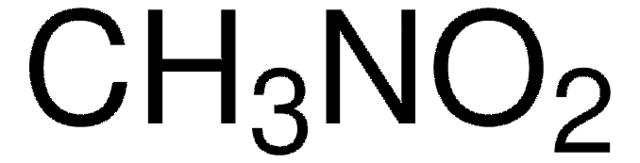Wichtige Dokumente
270423
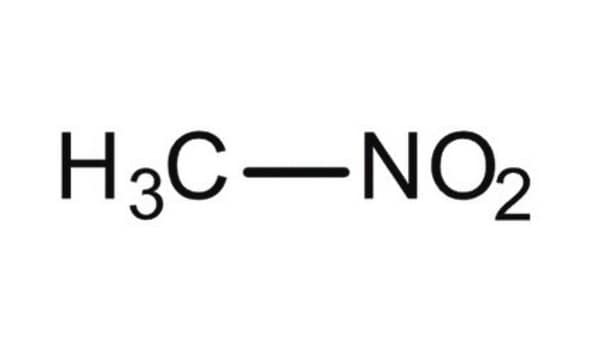
Nitromethan
suitable for HPLC, ≥96%
About This Item
Empfohlene Produkte
Dampfdichte
2.1 (vs air)
Qualitätsniveau
Dampfdruck
2.7 mmHg
Assay
≥96%
Form
liquid
Selbstzündungstemp.
784 °F
Expl.-Gr.
7.3 %, 33 °F
Methode(n)
HPLC: suitable
Verunreinigungen
<0.030% water
Brechungsindex
n20/D 1.382 (lit.)
pH-Wert
6.4 (20 °C, 0.01 g/L)
bp
101.2 °C (lit.)
mp (Schmelzpunkt)
−29 °C (lit.)
Dichte
1.127 g/mL at 25 °C (lit.)
λ
H2O reference
UV-Absorption
λ: 380 nm Amax: 1.00
λ: 386 nm Amax: 0.50
λ: 395 nm Amax: 0.20
λ: 400 nm Amax: 0.10
λ: 405 nm Amax: 0.05
λ: 430-700 nm Amax: 0.01
Anwendung(en)
food and beverages
SMILES String
C[N+]([O-])=O
InChI
1S/CH3NO2/c1-2(3)4/h1H3
InChIKey
LYGJENNIWJXYER-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Anwendung
- Asymmetric aza-Henry reaction toward trifluoromethyl β-nitroamines and biological investigation of their adamantane-type derivatives.: This study used nitromethane in asymmetric aza-Henry reactions to synthesize trifluoromethyl β-nitroamines, which were further investigated for their biological properties, showcasing the potential of nitromethane in advanced synthetic chemistry (Ren et al., 2024).
- Effect of Temperature on the Liquid Bridging Force while Maintaining Physical Stability in Solid-Liquid Mixed Fuel.: Nitromethane was analyzed in this research to understand its role in the stability of solid-liquid mixed fuels under varying temperatures, providing insights into the optimization of fuel formulations (Zhang et al., 2024).
- Generation of New Synthons for Synthesis Through Activation of Nitromethane.: This research demonstrated the activation of nitromethane to generate new synthons for synthetic applications, highlighting its versatility and importance in creating novel chemical entities (Wang et al., 2024).
- Towards Chemoenzymatic Syntheses of Both Enantiomers of Phosphoemeriamine.: The study explored the use of nitromethane in chemoenzymatic syntheses, enabling the production of both enantiomers of phosphoemeriamine, an important compound in chemical biology (Kiełbasiński et al., 2024).
- Rationally introducing non-canonical amino acids to enhance catalytic activity of LmrR for Henry reaction.: Nitromethane was employed in this study to investigate the enhancement of catalytic activity in the Henry reaction through the introduction of non-canonical amino acids, demonstrating its significance in enzyme catalysis research (Wang et al., 2024).
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Carc. 2 - Flam. Liq. 3 - Repr. 2
Lagerklassenschlüssel
4.1A - Other explosive hazardous materials
WGK
WGK 2
Flammpunkt (°F)
95.0 °F - closed cup
Flammpunkt (°C)
35 °C - closed cup
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Protokolle
GC Analysis of Class 2 Residual Solvents on OVI-G43
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.