Wichtige Dokumente
62122
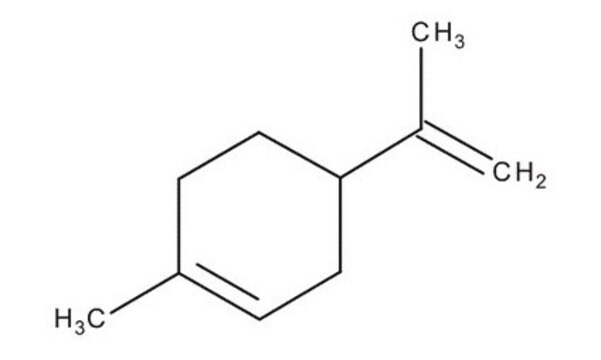
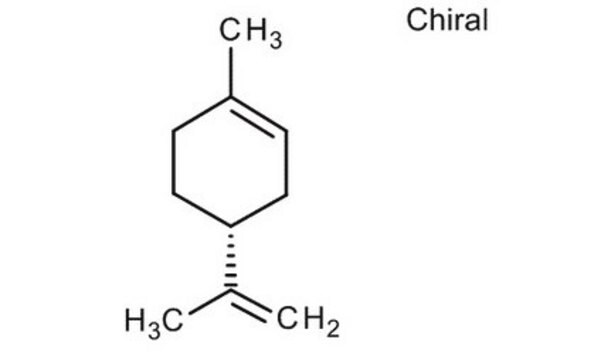
(R)-(+)-Limone
technical, ~90% (sum of enantiomers, GC)
Synonym(e):
(+)-p-Mentha-1,8-dien, (+)-Carven, (R)-4-Isopropenyl-1-methyl-1-cyclohexen
About This Item
Empfohlene Produkte
Dampfdichte
4.7 (vs air)
Qualitätsniveau
Dampfdruck
<3 mmHg ( 14.4 °C)
Qualität
technical
Assay
~90% (sum of enantiomers, GC)
Form
liquid
Optische Aktivität
[α]20/D +112±5°, c = 10% in ethanol
Expl.-Gr.
6.1 %
Grünere Alternativprodukt-Eigenschaften
Waste Prevention
Safer Solvents and Auxiliaries
Use of Renewable Feedstocks
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Brechungsindex
n20/D 1.473 (lit.)
bp
176-177 °C (lit.)
Löslichkeit
water: insoluble
Dichte
0.842 g/mL at 20 °C (lit.)
Grünere Alternativprodukt-Kategorie
Lagertemp.
2-8°C
SMILES String
CC(=C)[C@@H]1CCC(C)=CC1
InChI
1S/C10H16/c1-8(2)10-6-4-9(3)5-7-10/h4,10H,1,5-7H2,2-3H3/t10-/m0/s1
InChIKey
XMGQYMWWDOXHJM-JTQLQIEISA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
Anwendung
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Aquatic Acute 1 - Aquatic Chronic 1 - Asp. Tox. 1 - Flam. Liq. 3 - Skin Irrit. 2 - Skin Sens. 1
Lagerklassenschlüssel
3 - Flammable liquids
WGK
WGK 2
Flammpunkt (°F)
123.8 °F - closed cup
Flammpunkt (°C)
51 °C - closed cup
Persönliche Schutzausrüstung
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
Die passende Version wird nicht angezeigt?
Wenn Sie eine bestimmte Version benötigen, können Sie anhand der Lot- oder Chargennummer nach einem spezifischen Zertifikat suchen.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Chromatograms
suitable for GCUnser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.