700023P
Avanti
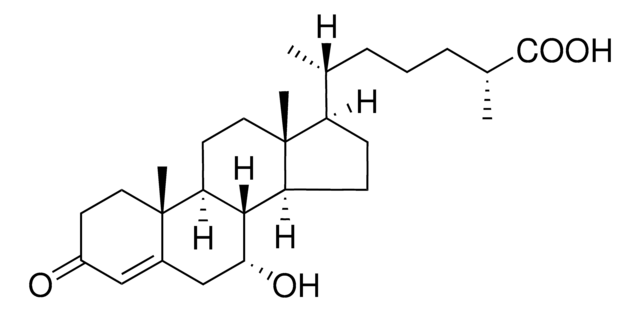
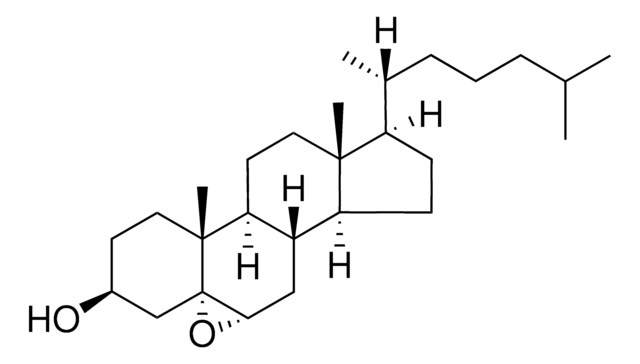
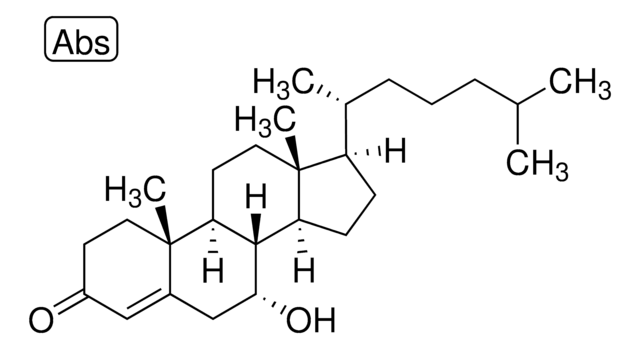
7α,27-dihydroxy-4-cholesten-3-one
Avanti Research™ - A Croda Brand
Synonym(e):
Cholest-4-en-3-one, 7,26-dihydroxy-, (7α,25R)-
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(2)
About This Item
Empirische Formel (Hill-System):
C27H44O3
CAS-Nummer:
Molekulargewicht:
416.64
UNSPSC-Code:
12352211
NACRES:
NA.25
Empfohlene Produkte
Form
powder
Verpackung
pkg of 1 × 1 mg (700023P-1mg)
Hersteller/Markenname
Avanti Research™ - A Croda Brand
Versandbedingung
dry ice
Lagertemp.
−20°C
Verwandte Kategorien
Allgemeine Beschreibung
7α,27-dihydroxy-4-cholesten-3-one synthesized by the hydroxylation of 27-hydroxycholesterol in the presence of the enzyme cholesterol 7 α-hydroxylase (CYP7A1) and is catabolized to bile acid. 7α,27-dihydroxy-4-cholesten-3-one is present majorly in fibroblasts and its conversion from cholesterol occurs in extrahepatic tissues.
Anwendung
7α,27-dihydroxy-4-cholesten-3-one may be used as a ligand to test its effect on Epstein-Barr virus-induced molecule 2 (EB12) activation in guanosine 5′-O-(3-thio)triphosphate ([35S] GTPγS) binding assay.
Biochem./physiol. Wirkung
7α,27-dihydroxy-4-cholesten-3-one is a suppressor of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase.
Verpackung
5 mL Amber Glass Screw Cap Vial (700023P-1mg)
Rechtliche Hinweise
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Lagerklassenschlüssel
11 - Combustible Solids
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Hepatic and extrahepatic dehydrogenation/isomerization of 5-cholestene-3beta, 7alpha-diol: localization of 3beta-hydroxy-Delta5-C27-steroid dehydrogenase in pig tissues and subcellular fractions
Furster C
Biochimica et Biophysica Acta - Molecular and Cell Biology of Lipids, 1436(3), 343-353 (1999)
27-Hydroxylated Low Density Lipoprotein (LDL) Cholesterol Can Be Converted to 7alpha, 27-Dihydroxy-4-cholesten-3-one (Cytosterone) before Suppressing Cholesterol Production in Normal Human Fibroblasts EVIDENCE THAT AN ALTERED METABOLISM OF LDL CHOLESTEROL
Axelson M and Larsson O
The Journal of Biological Chemistry, 271(22), 12724-12736 (1996)
Identification of structural motifs critical for epstein-barr virus-induced molecule 2 function and homology modeling of the ligand docking site
Zhang L, et al.
Molecular Pharmacology, 82(6), 1094-1103 (2012)
On the substrate specificity of human CYP27A1: implications for bile acid and cholestanol formation.
Maria Norlin et al.
Journal of lipid research, 44(8), 1515-1522 (2003-06-05)
The mitochondrial sterol 27-hydroxylase (CYP27A1) is required for degradation of the C27-sterol side chain in bile acid biosynthesis. CYP27A1 seems, however, to have roles beyond this, as illustrated by patients with a deficient sterol 27-hydroxylase due to mutations of the
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.