Alle Fotos(2)
Wichtige Dokumente
T90301
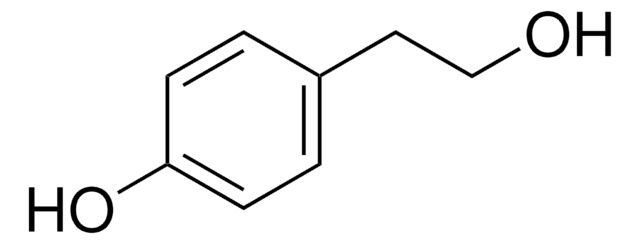
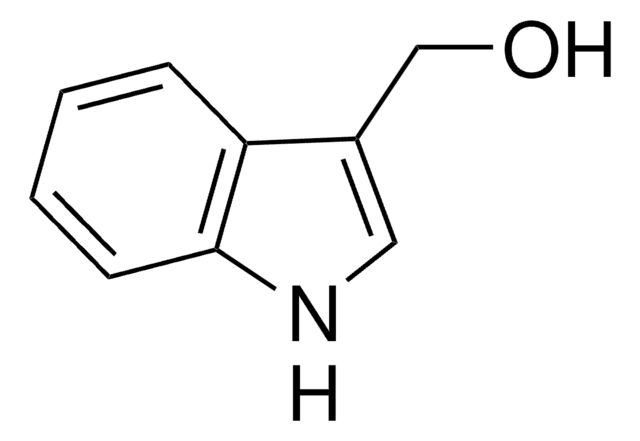
3-(2-Hydroxyethyl)-indol
97%
Synonym(e):
3-Indolethanol, ‘Tryptophol’
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(2)
About This Item
Empirische Formel (Hill-System):
C10H11NO
CAS-Nummer:
Molekulargewicht:
161.20
Beilstein:
125553
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
97%
mp (Schmelzpunkt)
56-59 °C (lit.)
SMILES String
OCCc1c[nH]c2ccccc12
InChI
1S/C10H11NO/c12-6-5-8-7-11-10-4-2-1-3-9(8)10/h1-4,7,11-12H,5-6H2
InChIKey
MBBOMCVGYCRMEA-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Anwendung
Reactant for preparation of:
- Inhibitors of the C-terminal domain of RNA polymerase II and their antitumor activities
- Anti-HIV-1 agents
- Inhibitors of Protein-Protein Interactions
- Partial agonists of the serotonin 5-HT1A receptor
- Growth hormone secretagogues
- Vascular endothelial growth factor (VEGF) inhibitors
- A2B adenosine receptor ligands
- Potential detoxification inhibitors of the crucifer phytoalexin brassinin
- Inhibitors of interleukine 6
- Dual binding site acetylcholinesterase inhibitors
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Olivier Vandeputte et al.
Applied and environmental microbiology, 71(3), 1169-1177 (2005-03-05)
The role and metabolism of indole-3-acetic acid in gram-negative bacteria is well documented, but little is known about indole-3-acetic acid biosynthesis and regulation in gram-positive bacteria. The phytopathogen Rhodococcus fascians, a gram-positive organism, incites diverse developmental alterations, such as leafy
Richard Pozdrik et al.
Journal of agricultural and food chemistry, 54(17), 6123-6129 (2006-08-17)
The disappearance of riboflavin absorbance at 445 nm from beers or model beers on light exposure is directly linked to light-struck character formation. The addition of (+)-catechin, (-)-epicatechin, tryptophol, or ascorbic acid was able to reduce, but not stop, absorbance
Dina Morshedi et al.
The FEBS journal, 274(24), 6415-6425 (2007-11-22)
Amyloid aggregation of polypeptides is related to a growing number of pathologic states known as amyloid disorders. There is a great deal of interest in developing small molecule inhibitors of the amyloidogenic processes. In the present article, the inhibitory effects
Xuqing Zhang et al.
Organic letters, 7(10), 2043-2046 (2005-05-07)
The tetrahydro-pyrano[3,4-b]indoles 6 were synthesized from 2-(2-trimethylsilanyl-1H-indol-3-yl)-ethanols 5 and various ketones or aldehydes through silicon-directed oxa-Pictet-Spengler cyclizations. An unusual reaction led to the dimeric products 7 when some of 5 was treated with acetone using BF(3) as the catalyst.
Ivan Kosalec et al.
Arhiv za higijenu rada i toksikologiju, 62(1), 41-49 (2011-03-23)
Tryptophol is an aromatic alcohol and secondary metabolite of the opportunistic fungus Candida albicans. Although its toxicity profile at cell level has been poorly investigated, recent data point to cytotoxic, cytostatic, and genotoxic effects in lymphocytes and the induction of
Global Trade Item Number
| SKU | GTIN |
|---|---|
| T90301-5G | 4061837380341 |
| T90301-1G |
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.