D5392
Dithiodiglykolsäure
≥98%
Synonym(e):
2,2′-Dithiodiacetic acid
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
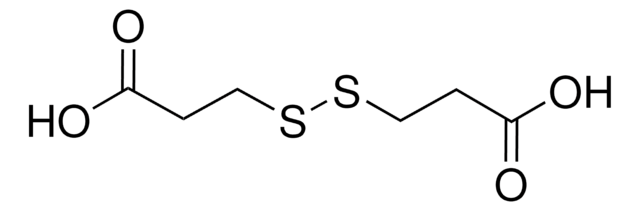
C4H6O4S2
CAS-Nummer:
Molekulargewicht:
182.22
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Assay
≥98%
Form
powder
Lagertemp.
−20°C
SMILES String
OC(=O)CSSCC(O)=O
InChI
1S/C4H6O4S2/c5-3(6)1-9-10-2-4(7)8/h1-2H2,(H,5,6)(H,7,8)
InChIKey
DLLMHEDYJQACRM-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Anwendung
- Enhancement of octacalcium phosphate via Dithiodiglycolic acid: Research on the interlayer expansion of octacalcium phosphate illustrates the forced oxidation of Dithiodiglycolic acid intercalated molecules within its interlayers, showcasing a novel application in material science and chemical engineering (Sugiura et al., 2023).
- Drug delivery systems: A study on redox-responsive self-assembly PEG nanoparticles enhanced by triptolide, where Dithiodiglycolic acid plays a critical role, demonstrates its potential in improving the efficacy of antitumor treatments, providing insights into pharmaceutical applications (Wang et al., 2018).
- Synthesis and medicinal applications: A comprehensive study on the synthesis and biological evaluation of Dithiodiglycolic acid derivatives via oxidative coupling of thiols highlights its utility in medicinal chemistry, offering new avenues for drug design and development (Bakulina et al., 2019).
- Redox-sensitive coordination polymers: The development of redox-sensitive nanoscale coordination polymers for drug delivery and cancer theranostics utilizes Dithiodiglycolic acid, providing significant advancements in cancer treatment strategies (Zhao et al., 2017).
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Hui-Yun Zhang et al.
International journal of pharmaceutics, 555, 270-279 (2018-11-25)
The anti-tumor efficacy of curcumin can be markedly improved by nano-drug self-delivery systems with high drug loading capacity and smart stimulus-triggered drug release in tumor cells. Herein, a type of novel, glutathione (GSH)-responsive, PEGylated prodrug nano-micelles (PPNMs) was prepared by
Huiyun Zhang et al.
International journal of pharmaceutics, 575, 118980-118980 (2020-01-04)
Cardiac glycosides (CGs) have been used to treat cancer for hundreds of years. However, the narrow therapeutic window and system toxicity have hindered their wide clinical applications. Herein, the small molecule prodrug strategy and nanotechnology were integrated into one drug
Shenwu Zhang et al.
Drug delivery, 24(1), 1460-1469 (2017-09-28)
Breast cancer leads to high mortality of women in the world. Docetaxel (DTX) has been widely applied as one of the first-line chemotherapeutic drugs for breast cancer therapy. However, the clinical outcome of DTX is far from satisfaction due to
Huiyun Zhang et al.
Drug development and industrial pharmacy, 46(11), 1800-1808 (2020-09-25)
Curcumin (CUR), a nontoxic natural compound with potent antitumor activity, was limited in clinical application due to its insolubility and exceedingly low bioavailability. In this study, a novel prodrug-nanoparticle (CSSV/TPGS-NPs) self-assembled by co-nanoprecipitation of CUR-s-s-vitamin E conjugate and d-alpha-tocopheryl polyethylene
Qingqing Xiong et al.
Frontiers in pharmacology, 9, 61-61 (2018-03-01)
Combination of doxorubicin with sorafenib (SF) was reported to be a promising strategy for treating hepatocellular carcinoma (HCC). In this study, we designed a reduction-responsive supramolecular nanosystem based on poly (ethylene glycol)-β-cyclodextrin (PEG-CD) and a disulfide-containing adamantine-terminated doxorubicin prodrug (AD)
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.