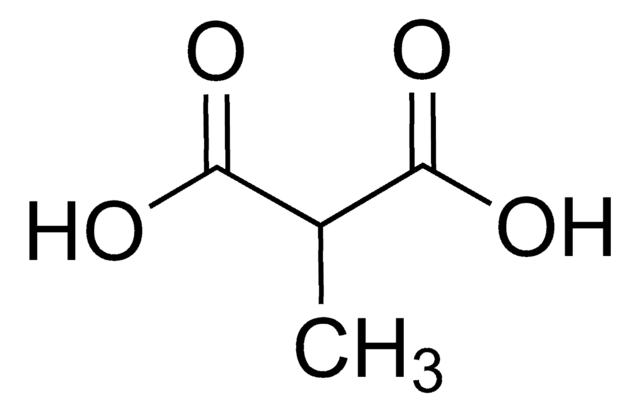
D168009
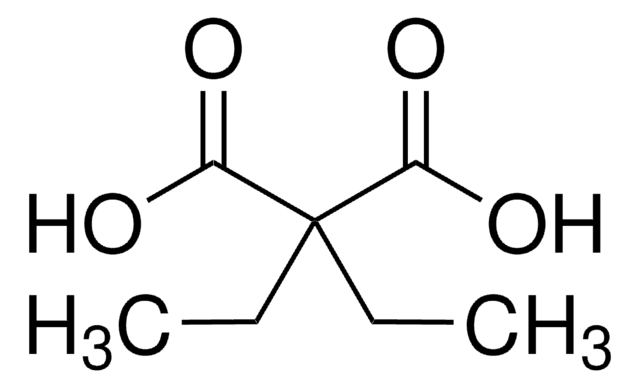
Dimethylmalonsäure
98%
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Lineare Formel:
(CH3)2C(COOH)2
CAS-Nummer:
Molekulargewicht:
132.11
Beilstein:
774375
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
98%
Form
crystals
mp (Schmelzpunkt)
191-193 °C (lit.)
SMILES String
CC(C)(C(O)=O)C(O)=O
InChI
1S/C5H8O4/c1-5(2,3(6)7)4(8)9/h1-2H3,(H,6,7)(H,8,9)
InChIKey
OREAFAJWWJHCOT-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
M Phillips et al.
Clinical chemistry, 38(1), 60-65 (1992-01-01)
We describe a method for the collection and microanalysis of the volatile organic compounds in human breath. A transportable apparatus supplies subjects with purified air and samples their alveolar breath; the volatile organic compounds are captured in an adsorptive trap
O Kniemeyer et al.
Applied and environmental microbiology, 65(8), 3319-3324 (1999-07-31)
The microbial capacity to degrade simple organic compounds with quaternary carbon atoms was demonstrated by enrichment and isolation of five denitrifying strains on dimethylmalonate as the sole electron donor and carbon source. Quantitative growth experiments showed a complete mineralization of
James R Harbridge et al.
Journal of magnetic resonance (San Diego, Calif. : 1997), 159(2), 195-206 (2002-12-17)
The effects of methyl rotation on electron spin-lattice relaxation times were examined by pulsed electron paramagnetic resonance for the major radicals in gamma-irradiated polycrystalline alpha-amino isobutyric acid, dimethyl-malonic acid, and L-valine. The dominant radical is the same in irradiated dimethyl-malonic
P A Frey et al.
Science (New York, N.Y.), 264(5167), 1927-1930 (1994-06-24)
Spectroscopic properties of chymotrypsin and model compounds indicate that a low-barrier hydrogen bond participates in the mechanism of serine protease action. A low-barrier hydrogen bond between N delta 1 of His57 and the beta-carboxyl group of Asp102 in chymotrypsin can
Elisabet Pires et al.
Physical chemistry chemical physics : PCCP, 22(42), 24351-24358 (2020-10-22)
The variation of the 31P chemical shift of triethylphosphine oxide in CDCl3 solution with a series of Brønsted acids at different molar ratios allows the determination of the value for the 1 : 1 species (δ1 : 1), which is much lower than the
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.


![Tris[2-(dimethylamino)ethyl]amin 97%](/deepweb/assets/sigmaaldrich/product/structures/695/792/ee0ff167-22a3-43a7-83a1-6c4908adf0ae/640/ee0ff167-22a3-43a7-83a1-6c4908adf0ae.png)