A95502
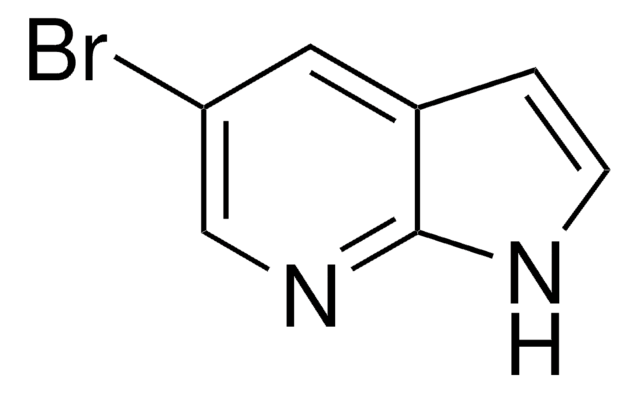
7-Azaindol
98%
Synonym(e):
1H-Pyrrolo(2,3-b)pyridin
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(3)
About This Item
Empirische Formel (Hill-System):
C7H6N2
CAS-Nummer:
Molekulargewicht:
118.14
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
98%
Form
solid
mp (Schmelzpunkt)
105-107 °C (lit.)
SMILES String
c1cnc2[nH]ccc2c1
InChI
1S/C7H6N2/c1-2-6-3-5-9-7(6)8-4-1/h1-5H,(H,8,9)
InChIKey
MVXVYAKCVDQRLW-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Anwendung
A heterocyclic molecule that can be utilized as a pharmaceutical building block
Starting material in a recent synthesis of azaserotonin.
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Alastair Donald et al.
Journal of medicinal chemistry, 50(10), 2289-2292 (2007-04-25)
6-phenylpurines were identified as novel, ATP-competitive inhibitors of protein kinase B (PKB/Akt) from a fragment-based screen and were rapidly progressed to potent compounds using iterative protein-ligand crystallography with a PKA-PKB chimeric protein. An elaborated lead compound showed cell growth inhibition
John J Caldwell et al.
Journal of medicinal chemistry, 51(7), 2147-2157 (2008-03-19)
Fragment-based screening identified 7-azaindole as a protein kinase B inhibitor scaffold. Fragment elaboration using iterative crystallography of inhibitor-PKA-PKB chimera complexes efficiently guided improvements in the potency and selectivity of the compounds, resulting in the identification of nanomolar 6-(piperidin-1-yl)purine, 4-(piperidin-1-yl)-7-azaindole, and
Xin Wang et al.
The Journal of organic chemistry, 71(10), 4021-4023 (2006-05-06)
A practical synthesis of a key pharmaceutical intermediate, 2-[(1H-pyrrolo[2,3-b]pyridine-4-yl)methylamino]-5-fluoronicotinic acid (1), is described. To introduce the aminomethyl moiety of 2 via a palladium-catalyzed cyanation/reduction sequence, a regioselective chlorination of 7-azaindole via the N-oxide was developed. A highly selective monodechlorination of
Pei-Wen Wu et al.
Journal of the American Chemical Society, 128(45), 14426-14427 (2006-11-09)
We report the synthesis of 3-(2-aminoethyl)-5-ol-1H-pyrrolo[2,3-b]pyridine (7-azaserotonin), which may potentially serve as an agonist or antagonist of serotonin receptors. In alcohols, the solvent (e.g., ethanol) catalyzed proton-transfer reaction takes place for 7-azaserotonin in the excited state, resulting in dual emission.
Bruno Aleksander Martek et al.
Drug testing and analysis, 11(4), 617-625 (2019-02-08)
The high frequency of the synthetic cannabinoid receptor agonists (SCRAs) emergence renders this group of new psychoactive compounds particularly demanding in terms of detection, identification, and responding. Without the available reference material, one of the specific problems is differentiation and
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.![1H-pyrrolo[3,2-c]pyridine AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/291/250/879d6f32-75bf-4a4a-a366-18cbd62dcc37/640/879d6f32-75bf-4a4a-a366-18cbd62dcc37.png)
![1H-Pyrrolo[2,3-c]pyridine AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/332/393/b23058ac-c477-42b5-9890-dc5c2af5cec7/640/b23058ac-c477-42b5-9890-dc5c2af5cec7.png)
![1H-pyrrolo[3,2-b]pyridine AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/276/658/7030377c-695d-4006-bbf3-fe99211bb7e7/640/7030377c-695d-4006-bbf3-fe99211bb7e7.png)