Wichtige Dokumente
901250
2-(Pyridin-2-yl)quinolin-8-amine dihydrochloride
Synonym(e):
PAQ directing group
About This Item
Empfohlene Produkte
Assay
≥95%
Form
powder or crystals
Eignung der Reaktion
reaction type: C-C Bond Formation
reagent type: catalyst
reagent type: ligand
reaction type: C-H Activation
Anwendung
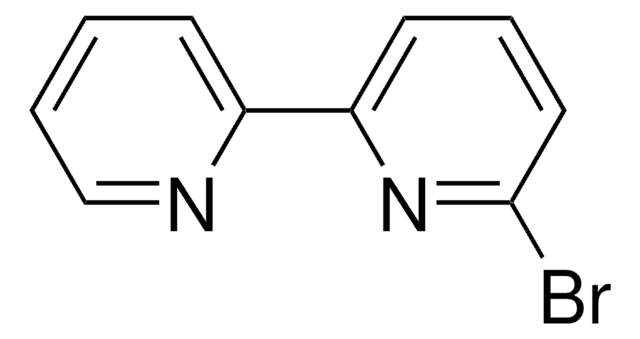
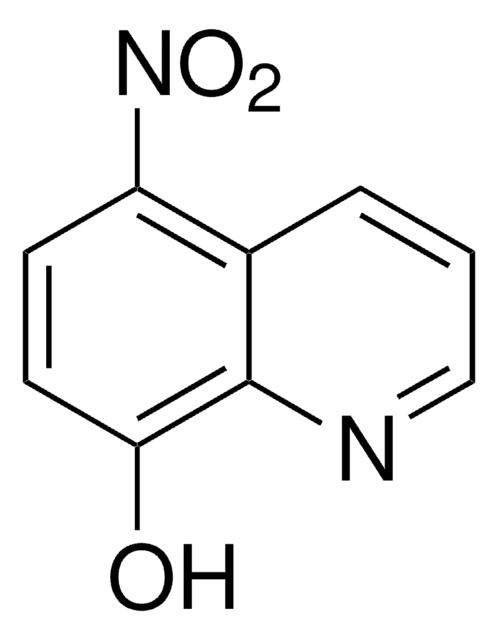
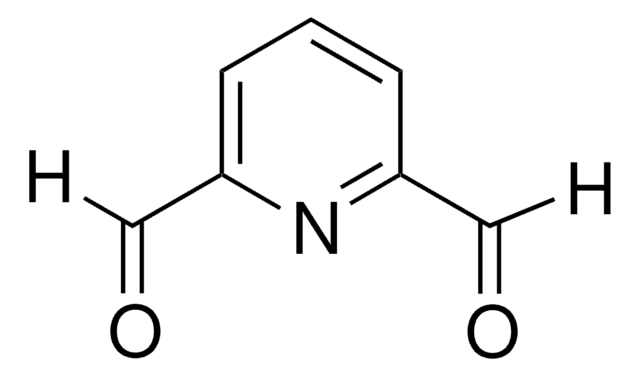
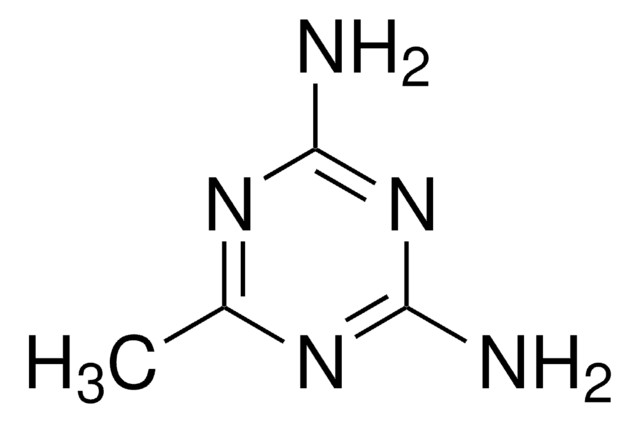
Ähnliches Produkt
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
Die passende Version wird nicht angezeigt?
Wenn Sie eine bestimmte Version benötigen, können Sie anhand der Lot- oder Chargennummer nach einem spezifischen Zertifikat suchen.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Verwandter Inhalt
The Engle lab strives to invent novel catalytic alkene and alkyne functionalization methods to expedite organic synthesis. These transformations offer a powerful platform for conversion of simple, abundant, and planar starting materials into densely functionalized, stereochemically complex products in a single step. To this end, the Engle lab has developed various substrate directivity strategies in which native functional groups can be temporarily masked with auxiliaries that are capable of reversibly binding the metal catalyst, thereby enhancing kinetic reactivity, suppressing unwanted side reactions, and facilitating high selectivity. The Engle lab works with us to make synthetically enabling directing groups, catalysts, and ligands readily available to the synthetic community for reaction discovery and small-molecule synthesis.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.![[2,2′-Bipyridine]-6-carboxylic acid hydrochloride](/deepweb/assets/sigmaaldrich/product/structures/130/786/b0e8142b-94b7-4abd-8a9a-0db3780a9f3d/640/b0e8142b-94b7-4abd-8a9a-0db3780a9f3d.png)