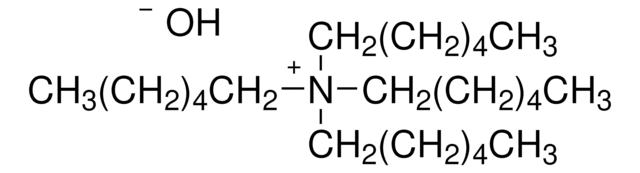88005
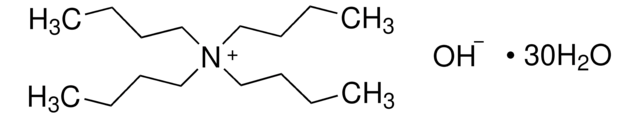
Tetrapentylammoniumhydroxid -Lösung
~20% in H2O (T)
About This Item
Empfohlene Produkte
Form
solution
Konzentration
~20% in H2O (T)
Verunreinigungen
≤0.05% halides (as bromide)
Anionenspuren
sulfate (SO42-): ≤500 mg/kg
Funktionelle Gruppe
amine
SMILES String
[OH-].CCCCC[N+](CCCCC)(CCCCC)CCCCC
InChI
1S/C20H44N.H2O/c1-5-9-13-17-21(18-14-10-6-2,19-15-11-7-3)20-16-12-8-4;/h5-20H2,1-4H3;1H2/q+1;/p-1
InChIKey
JVOPCCBEQRRLOJ-UHFFFAOYSA-M
Verwandte Kategorien
Anwendung
- As a model compound in the studies of the effects of quaternary ammonium salts on the formation of clathrate hydrates of methane.
- To maintain the pH of the aqueous phase to study the adsorption dynamics of nanoparticles at fluid interfaces.
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Skin Corr. 1B
Lagerklassenschlüssel
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.