Alle Fotos(1)
Wichtige Dokumente
740683
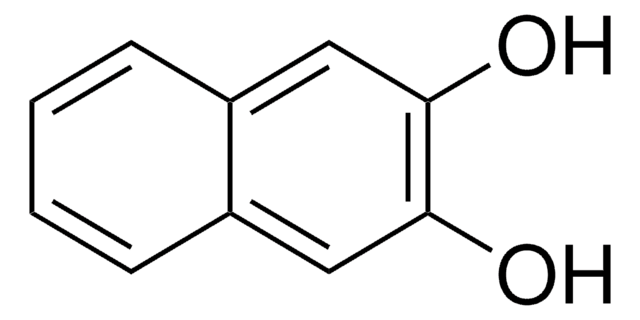
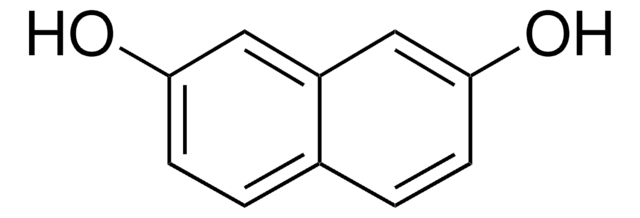
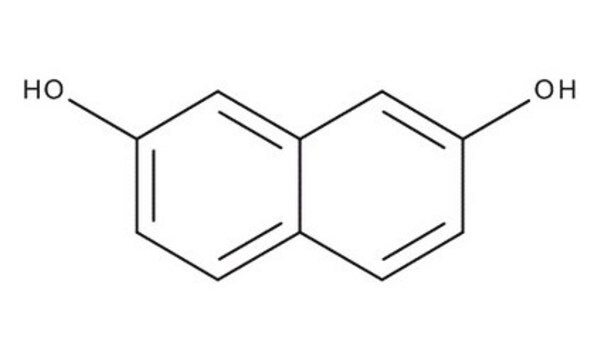
1,8-Dihydroxynaphthalin
95%
Synonym(e):
1,8-Naphthalindiol
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C10H8O2
CAS-Nummer:
Molekulargewicht:
160.17
Beilstein:
2044947
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Assay
95%
Form
solid
mp (Schmelzpunkt)
137-143 °C
Lagertemp.
2-8°C
SMILES String
Oc1cccc2cccc(O)c12
InChI
1S/C10H8O2/c11-8-5-1-3-7-4-2-6-9(12)10(7)8/h1-6,11-12H
InChIKey
OENHRRVNRZBNNS-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Anwendung
1,8-Dihydroxynaphthalene (DHN) can be used as:
- An intermediate in the preparation of benzo analogs of spiromamakone A.
- A starting material to synthesize naphthopyran derivatives.
- An intermediate in the total synthesis of palmarumycin CP17 analogs.
Signalwort
Danger
H-Sätze
P-Sätze
Gefahreneinstufungen
Eye Dam. 1
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
E Thines et al.
The Journal of antibiotics, 51(4), 387-393 (1998-06-19)
From submerged cultures of Scytalidium sp. 36-93, ten metabolites were isolated due to their effects on dihydroxynaphthalene (DHN) or DOPA melanin biosynthesis. Four of the compounds, scytalols A (1a), B (1b), C (2) and D (3), are new secondary metabolites
Pigment biosynthesis and virulence.
A A Brakhage et al.
Contributions to microbiology, 2, 205-215 (1999-10-16)
Hong Jiang et al.
Gene, 602, 8-15 (2016-11-16)
A PKS1 gene responsible for the melanin biosynthesis and a NPG1 gene in Aureobasidium melanogenum XJ5-1 were cloned and characterized. An ORF of the PKS1 gene encoding a protein with 2165 amino acids contained 6495bp while an ORF of the
Shao Yu Lin et al.
Molecular plant-microbe interactions : MPMI, 25(12), 1552-1561 (2012-09-01)
Both Colletotrichum and Magnaporthe spp. develop appressoria pigmented with melanin, which is essential for fungal pathogenicity. 1,8-Dihydroxynaphthalene (1,8-DHN) is believed to be polymerized to yield melanin around the appresorial cell wall through the oxidative activity of laccases. However, no 1,8-DHN
H F Tsai et al.
The Journal of biological chemistry, 276(31), 29292-29298 (2001-05-15)
Chain lengths and cyclization patterns of microbial polyketides are generally determined by polyketide synthases alone. Fungal polyketide melanins are often derived from a pentaketide 1,8-dihydroxynaphthalene, and pentaketide synthases are used for synthesis of the upstream pentaketide precursor, 1,3,6,8-tetrahydroxynaphthalene (1,3,6,8-THN). However
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.