673854
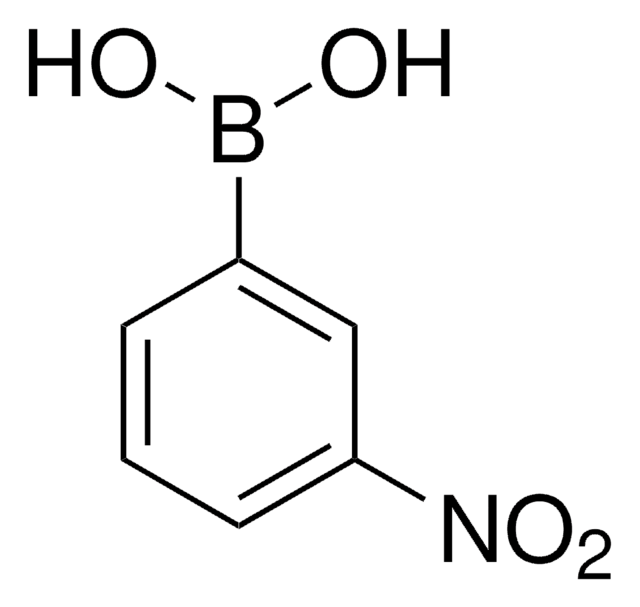
4-Nitrophenylboronsäure
≥95.0%
Synonym(e):
4-Nitrobenzeneboronic acid, p-Nitrophenylboronic acid, p-nitro-benzeneboronic acid
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(3)
About This Item
Lineare Formel:
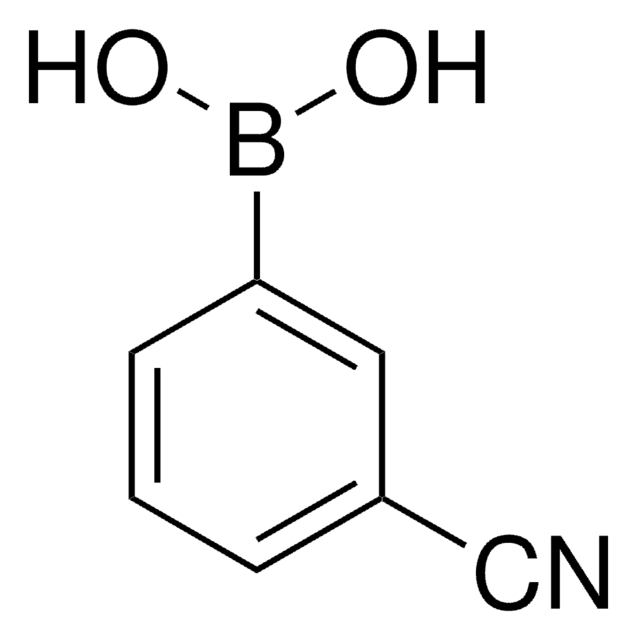
(O2N)C6H4(B(OH)2)
CAS-Nummer:
Molekulargewicht:
166.93
MDL-Nummer:
UNSPSC-Code:
12352103
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
≥95.0%
Form
solid
mp (Schmelzpunkt)
285-290 °C (dec.)
SMILES String
OB(O)c1ccc(cc1)[N+]([O-])=O
InChI
1S/C6H6BNO4/c9-7(10)5-1-3-6(4-2-5)8(11)12/h1-4,9-10H
InChIKey
NSFJAFZHYOAMHL-UHFFFAOYSA-N
Anwendung
Reagent used for
Reagent used in Preparation of
- Ligand-free palladium-catalyzed Suzuki-Miyaura cross-couplings
- Ruthenium catalyzed direct arylation of benzylic sp3 carbons of acyclic amines
- Diels-Alder or C-H activation reactions
- Regioselective Suzuki-Miyaura coupling and tandem palladium-catalyzed intramolecular aminocarbonylation and annulations
- N-arylation of phenylurea using copper acetylacetonate catalyst
- Environmentally benign one-pot synthesis through a double arylation process
- Copper-mediated cyanations
- copper-catalyzed arylations
- Regioselective glycosylations
- Suzuki couplings followed by arylations
- X-ray absorption on rhodium-grafted hydrotalcite catalyst for heterogeneous 1,4-addition reaction of organoboron reagents to electron deficient olefins
Reagent used in Preparation of
- Combretastatin analogs as potential antitumor agents
- Human immunodeficiency virus (HIV) protease inhibitors with antiviral activities against drug-resistant viruses
Sonstige Hinweise
May contain varying amounts of anhydride
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Rhodium-grafted hydrotalcite catalyst for heterogeneous 1,4-addition reaction of organoboron reagents to electron deficient olefins
Motokura, K.; et al.
Green Chemistry, 13, 2416-2422 (2011)
An efficient copper-catalyzed one-pot synthesis of diaryl thioethers by coupling of arylboronic acids with potassium ethyl xanthogenate under mild conditions
Wang, L.; et al.
Synlett, 20, 3041-3045 (2011)
An efficient access to 2,3-diarylimidazo[1,2-a]pyridines via imidazo[1,2-a]pyridin-2-yl triflate through a Suzuki cross-coupling reaction-direct arylation sequence
Marhadour, S.; et al.
Tetrahedron Letters, 53, 297-300 (2012)
Abdallah Hamze et al.
ChemMedChem, 6(12), 2179-2191 (2011-10-13)
A novel class of isocombretastatin A-4 (isoCA-4) analogues with modifications at the 3'-position of the B-ring by replacement with C-linked substituents was studied. Exploration of the structure-activity relationships of theses analogues led to the identification of several compounds that exhibit
Tetrahedron, 63, 6131-6131 (2007)
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.