Alle Fotos(1)
Wichtige Dokumente
539112
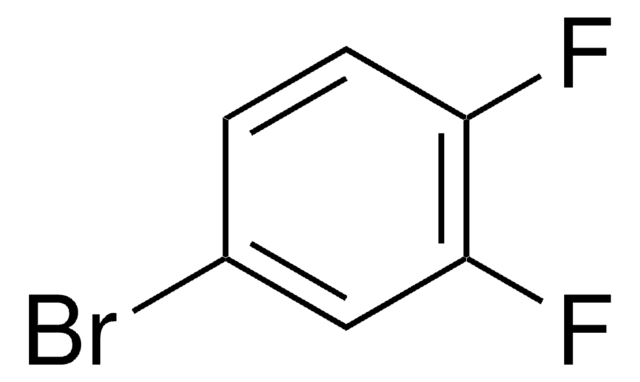
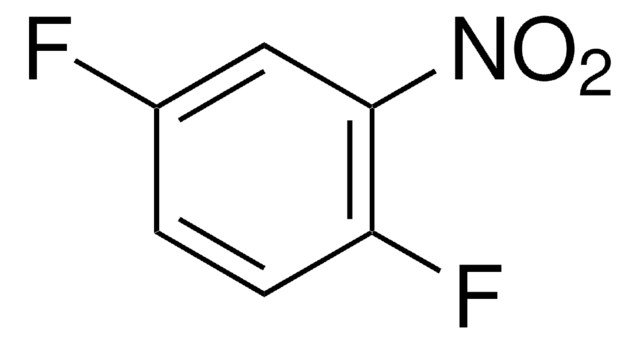
4-Brom-1-fluor-2-nitrobenzol
96%
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Lineare Formel:
BrC6H3(F)NO2
CAS-Nummer:
Molekulargewicht:
220.00
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
96%
Brechungsindex
n20/D 1.575 (lit.)
bp
240-241 °C (lit.)
mp (Schmelzpunkt)
18-19 °C (lit.)
Dichte
1.786 g/mL at 25 °C (lit.)
Funktionelle Gruppe
bromo
fluoro
nitro
SMILES String
[O-][N+](=O)c1cc(Br)ccc1F
InChI
1S/C6H3BrFNO2/c7-4-1-2-5(8)6(3-4)9(10)11/h1-3H
InChIKey
UQEANKGXXSENNF-UHFFFAOYSA-N
Allgemeine Beschreibung
4-Bromo-1-fluoro-2-nitrobenzene undergoes Sonogashira reaction with 2-fluoronitrobenzene to afford predominantly the bromo displacement product.
Anwendung
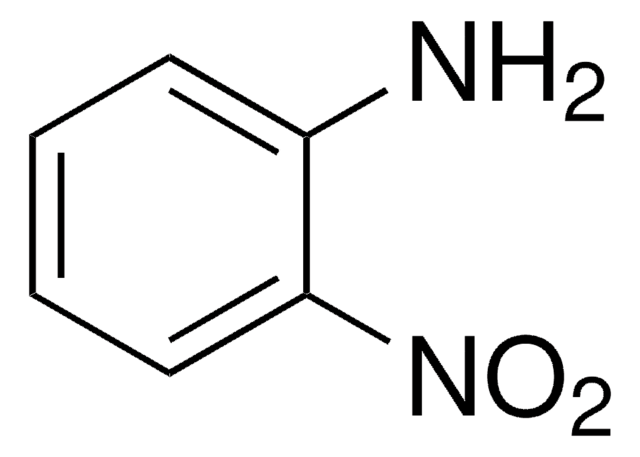
Used in the synthesis of anti-inflammatory agents.
4-Bromo-1-fluoro-2-nitrobenzene may be used in the synthesis of:
- 6-bromo-1H-benzo[d][1,2,3]triazol-1-ol
- 2-(4-bromo-2-nitrophenylamino)-5-methylthiophene-3-carbonitrile
- dibenzoxazepine analog, as potent sodium channel blocker
- 4-(4-bromo-2-nitrophenyl)piperazine-1-carboxylic acid tert-butylester
Lagerklassenschlüssel
10 - Combustible liquids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Katie M Lutker et al.
Crystal growth & design, 8(1), 136-139 (2008-01-01)
Bis(5-methyl-2-[(2-nitrophenyl)amino]-3-thiophenecarbonitrilyl)acetylene, a derivative of the highly polymorphic compound 5-methyl-2-[(2-nitrophenyl)amino]-3-thiophenecarbonitrile (ROY) that possesses two chromophores electronically coupled through a triple bond, was found to be trimorphic. Structural data for two of these forms indicates that symmetry is maintained in one structure
Patrick L DeRoy et al.
Organic letters, 9(14), 2741-2743 (2007-06-08)
The nucleophilic aromatic substitution reaction between electron-deficient aryl fluorides and terminal alkynes is shown to be efficiently promoted by sodium bis(trimethylsilyl)amide as a base. Moderate to excellent yields of 2-ethynylnitrobenzene products can be obtained under mild conditions.
Erik Rytter Ottosen et al.
Journal of medicinal chemistry, 46(26), 5651-5662 (2003-12-12)
We wish to report the synthesis and structure-activity relationship (SAR) of a series of 4-aminobenzophenones, as a novel compound class with high antiinflammatory activity. Our initial lead, (4-[(2-aminophenyl)amino]phenyl)(phenyl)methanone (3), was systematically optimized and resulted in compounds that potently inhibited the
Stephen M Lynch et al.
Bioorganic & medicinal chemistry letters, 25(1), 43-47 (2014-12-04)
We have identified two related series of dibenzazepine and dibenzoxazepine sodium channel blockers, which showed good potency on Nav1.7 in FLIPR-based and electrophysiological functional assays.
Tomoki Kawai et al.
Nuclear medicine and biology, 40(5), 705-709 (2013-05-28)
As a first trial for in vivo imaging of β-secretase (BACE1) in Alzheimer's disease brain, we applied a novel non-peptidergic small molecule which has high affinity to the enzyme, naphthalene-1-carboxylic acid (3'-chloro-4'-fluoro-4-piperazin-1-yl-biphenyl-3-yl)amide (NCFB) into positron emission tomography (PET) probe. In
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.![[1,1′-Bis(diphenylphosphino)ferrocen]dichlorpalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-Bis(diphenylphosphin)ferrocen]dichlorpalladium(II), Komplex mit Dichlormethan](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)