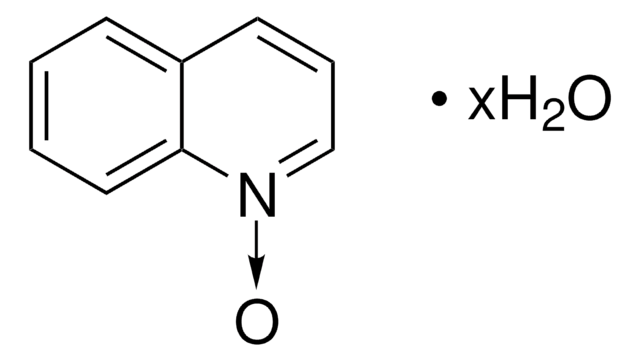
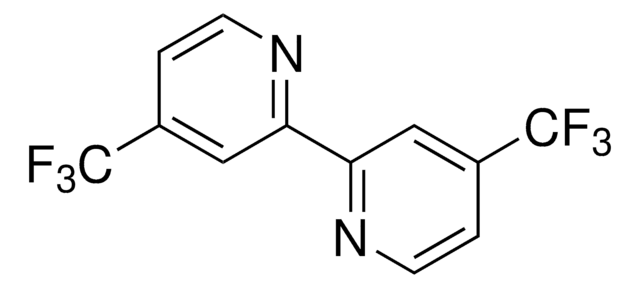
Alle Fotos(1)
Wichtige Dokumente
520268
4,4′,4″-Tri-tert-Butyl-2,2′:6′,2″-terpyridin
95%
Synonym(e):
2,6-Bis[4-(tert-butyl)pyridin-2-yl)-4-(tert-butyl)pyridine
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C27H35N3
CAS-Nummer:
Molekulargewicht:
401.59
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Assay
95%
Verunreinigungen
oligomers of tert-butyl-terpyridine
mp (Schmelzpunkt)
215-217 °C (lit.)
SMILES String
CC(C)(C)c1ccnc(c1)-c2cc(cc(n2)-c3cc(ccn3)C(C)(C)C)C(C)(C)C
InChI
1S/C27H35N3/c1-25(2,3)18-10-12-28-21(14-18)23-16-20(27(7,8)9)17-24(30-23)22-15-19(11-13-29-22)26(4,5)6/h10-17H,1-9H3
InChIKey
QMABMHJGSFUTPF-UHFFFAOYSA-N
Allgemeine Beschreibung
4,4′,4″-Tri-tert-Butyl-2,2′:6′,2″-terpyridine is a terpyridine (terpy) ligands that is commonly used in organic synthesis due to its role in coordination chemistry, particularly in the formation of metal complexes such as Zn(II), Cd, and lanthanide-cadmium pentafluorobenzoate complexes.
Anwendung
4,4′,4-Tri-tert-Butyl-2,2′:6′,2-terpyridine can be used as a ligand:
- In the synthesis of methylated alkanes and ketones via Ni-catalyzed methylation of unactivated alkyl halides and acid chlorides.
- In Ni-catalyzed reductive dimerization reaction.
- In allylic defluorinative reductive cross-coupling reaction in the presence of Ni as a catalyst.
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Zhuye Liang et al.
Organic letters, 16(21), 5620-5623 (2014-10-22)
Methylation of unactivated alkyl halides and acid chlorides under Ni-catalyzed reductive coupling conditions led to efficient formation of methylated alkanes and ketones using methyl p-methyl tosylate as the methylation reagent. Moderate to excellent coupling yields as well as excellent functional
Xiao-Yu Lu et al.
Organic & biomolecular chemistry, 18(19), 3674-3678 (2020-05-08)
A nickel-catalyzed defluorinative reductive cross-coupling of trifluoromethyl alkenes with epoxides has been developed. Various substituted trifluoromethyl alkenes and epoxides were found to be suitable reaction substrates. This reaction enabled C(sp3)-C(sp3) bond construction through allylic defluorinative cross-coupling of trifluoromethyl alkenes under
Michael R Prinsell et al.
Chemical communications (Cambridge, England), 46(31), 5743-5745 (2010-06-29)
The first general method for the reductive dimerization of alkyl halides, alkyl mesylates, alkyl trifluoroacetates, and allylic acetates is reported which proceeds with low catalyst loading (0.5 to 5 mol%), generally high yields (80% ave yield), and good functional-group tolerance.
Cheng-Pan Zhang et al.
Journal of the American Chemical Society, 135(22), 8141-8144 (2013-05-23)
Mechanistic proposals for nickel-catalyzed coupling reactions often invoke five-coordinate alkyl- or aryl-bound Ni(II) and/or high-valent nickel(III) species, but because of their reactive nature, they have been difficult to study and fingerprint. In this work, we invoked the stabilizing properties of
Gavin D Jones et al.
Journal of the American Chemical Society, 128(40), 13175-13183 (2006-10-05)
The ability of the terpyridine ligand to stabilize alkyl complexes of nickel has been central in obtaining a fundamental understanding of the key processes involved in alkyl-alkyl cross-coupling reactions. Here, mechanistic studies using isotopically labeled (TMEDA)NiMe(2) (TMEDA = N,N,N',N'-tetramethylethylenediamine) have
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.




![2,6-Bis[(4R)-(+)-isopropyl-2-oxazolin-2-yl]pyridin 99%](/deepweb/assets/sigmaaldrich/product/structures/349/609/8673c46e-368a-47a6-a9bd-52bbe13d490a/640/8673c46e-368a-47a6-a9bd-52bbe13d490a.png)