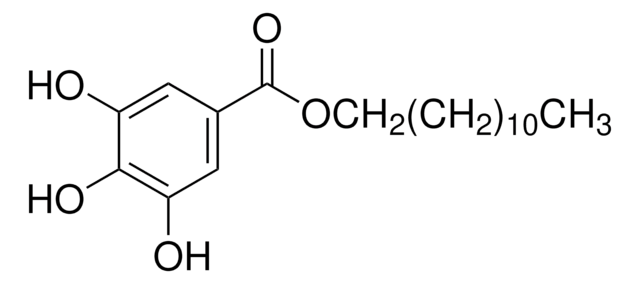
48700
Octylgallat
antioxidant, ≥99.0% (HPLC)
Synonym(e):
Octyl 3,4,5-trihydroxybenzoate, n-Octyl gallate
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
≥99.0% (HPLC)
mp (Schmelzpunkt)
101-103 °C
101-104 °C (lit.)
Löslichkeit
soluble 2.5%, clear, colorless (AcOH (MEOH))
Funktionelle Gruppe
ester
SMILES String
CCCCCCCCOC(=O)c1cc(O)c(O)c(O)c1
InChI
1S/C15H22O5/c1-2-3-4-5-6-7-8-20-15(19)11-9-12(16)14(18)13(17)10-11/h9-10,16-18H,2-8H2,1H3
InChIKey
NRPKURNSADTHLJ-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Allgemeine Beschreibung
Anwendung
Haftungsausschluss
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Oral - Skin Sens. 1
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 1
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.