429120
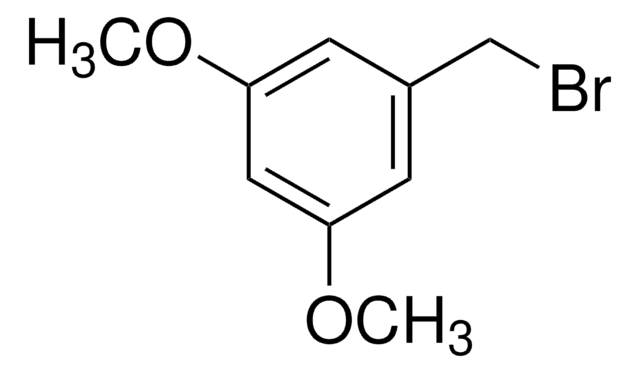
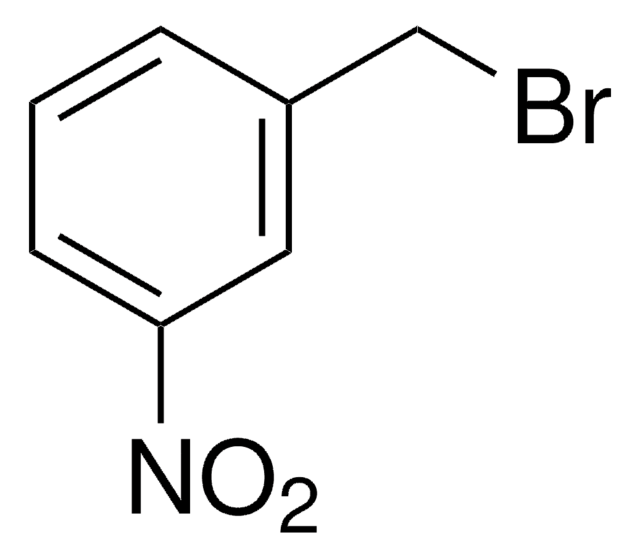
3-Methoxybenzylbromid
98%
Synonym(e):
1-Brommethyl-3-methoxybenzol
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Lineare Formel:
CH3OC6H4CH2Br
CAS-Nummer:
Molekulargewicht:
201.06
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
98%
Form
liquid
Brechungsindex
n20/D 1.575 (lit.)
bp
152 °C (lit.)
Dichte
1.436 g/mL at 25 °C (lit.)
SMILES String
COc1cccc(CBr)c1
InChI
1S/C8H9BrO/c1-10-8-4-2-3-7(5-8)6-9/h2-5H,6H2,1H3
InChIKey
ZKSOJQDNSNJIQW-UHFFFAOYSA-N
Allgemeine Beschreibung
3-Methoxybenzyl bromide is a benzyl bromide derivative.
Anwendung
3-Methoxybenzyl bromide (1-bromomethyl-3-methoxybenzene) may be used in the diastereoselective alkylation of vicinal dianions of chiral succinic acid derivatives.
It may be used in the synthesis of the following:
It may be used in the synthesis of the following:
- 6-(3-methoxyphenyl)-hexane-2,4-dione
- N-(3-methoxybenzyl)-N-(1-methyl-1-phenylethyl)-amine
- 2-(3-methoxybenzyl)-3-[(1R)-7,7-dimethyl-2-oxobicyclo[2.2.1]hept-1-yl]-(3S)-2-thionia-bicyclo [2.2.1]- heptane tetrafluoroborate
- 1-(3-methoxybenzyl)-5-(1-methyl-1H-imidazol-5-yl)-1H-1,2,3-triazole
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Eye Dam. 1 - Skin Corr. 1B
Lagerklassenschlüssel
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flammpunkt (°F)
235.4 °F - closed cup
Flammpunkt (°C)
113 °C - closed cup
Persönliche Schutzausrüstung
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Yang Zhang et al.
The Journal of organic chemistry, 71(12), 4516-4520 (2006-06-06)
A mild protocol for the conversion of beta-ketoesters and beta-diketones to carboxylic acids with use of CAN in CH3CN is described. The method is compatible with a number of functional groups, and can generate carboxylic acids under neutral conditions at
Highly diastereoselective alkylation of vicinal dianions of chiral succinic acid derivatives: a new general strategy to (R)-?-arylmethyl-?-butyrolactones.
Pohmakotr M, et al.
Tetrahedron Letters, 45(22), 4315-4318 (2004)
Synthesis of (-)-kainic acid using chiral lithium amides in an asymmetric dearomatizing cyclization.
Clayden J, et al.
Tetrahedron, 58(23), 4727-4733 (2002)
Application of sulfur ylide mediated epoxidations in the asymmetric synthesis of ?-hydroxy-d-lactones. Synthesis of a mevinic acid analogue and (+)-prelactone B.
Aggarwal VK, et al.
Tetrahedron Asymmetry, 60(43), 9725-9733 (2004)
Johan R Johansson et al.
The Journal of organic chemistry, 76(7), 2355-2359 (2011-03-11)
An experimentally simple sequential one-pot RuAAC reaction, affording 1,5-disubstituted 1H-1,2,3-triazoles in good to excellent yields starting from an alkyl halide, sodium azide, and an alkyne, is reported. The organic azide is formed in situ by treating the primary alkyl halide
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.