Alle Fotos(2)
Wichtige Dokumente
405418
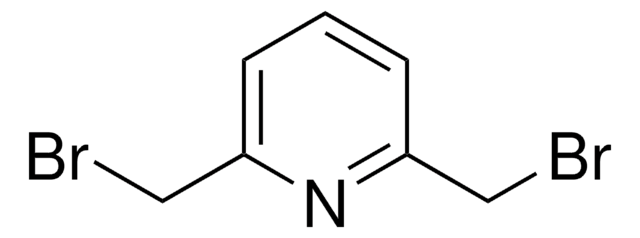
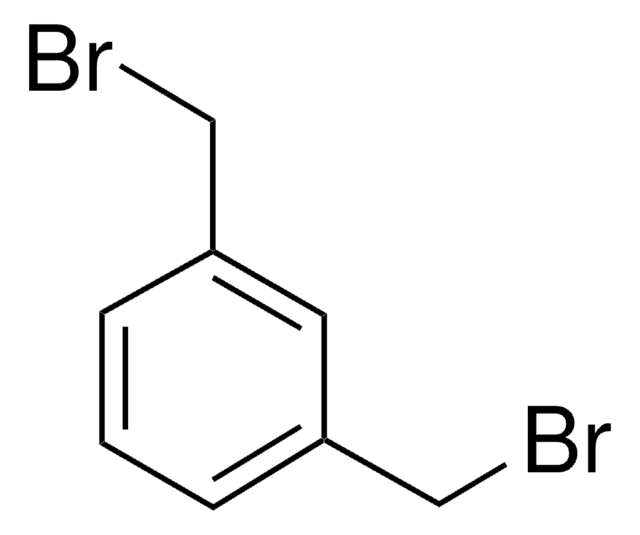
2,6-Bis(chlormethyl)pyridin
99%
Synonym(e):
α,α′-Dichlor-2,6-lutidin
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(2)
About This Item
Empirische Formel (Hill-System):
C7H7Cl2N
CAS-Nummer:
Molekulargewicht:
176.04
Beilstein:
116355
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Assay
99%
Form
solid
mp (Schmelzpunkt)
73-78 °C (lit.)
Funktionelle Gruppe
chloro
SMILES String
ClCc1cccc(CCl)n1
InChI
1S/C7H7Cl2N/c8-4-6-2-1-3-7(5-9)10-6/h1-3H,4-5H2
InChIKey
IWQNFYRJSVJWQA-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Allgemeine Beschreibung
2,6-Bis(chloromethyl)pyridine is a heterocyclic building block for the synthesis of a variety of pyridine derivatives. It coordinates with metal ions through N-atom to form complexes. The conformational flexibility of the bromomethyl arms makes it an ideal choice for the generation of macrocycles. 2,6-bis(chloromethyl)pyridine crystals are monoclinic with space group P21/c. Its synthesis has been reported. The FT-IR and FT-Raman spectra of 2,6-bis(chloromethyl)pyridine (BCMP) have been recorded in the regions 4000-400cm-1 and 3500-100cm-1, respectively.
Anwendung
2,6-Bis(chloromethyl)pyridine may be used in the following studies:
- Synthesis of a sensitive fluorescent chemosensor for Hg2+, composed of two aminonaphthalimide fluorophores and a receptor of 2,6-bis(aminomethyl)pyridine.
- Preparation of carbene pincer ligands, required for the preparation of palladium pincer carbene complex.
- Synthesis of 2-(di-tert-butylphosphinomethyl)-6-diethylaminomethyl)pyridine, PNN ligand.
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Iron (II) complexes based on electron-rich, bulky PNN-and PNP-type ligands.
Zhang J, et al.
Inorgorganica Chimica Acta, 359(6), 1955-1960 (2006)
Xiangfeng Guo et al.
Journal of the American Chemical Society, 126(8), 2272-2273 (2004-02-26)
A selective and sensitive fluorescent chemosensor for Hg2+, which was composed of two aminonaphthalimide fluorophores and a receptor of 2,6-bis(aminomethyl)pyridine, was synthesized through the reaction of 2,6-bis(chloromethyl)pyridine and N-[2-(2-hydroxyethoxy)ethyl]-4-piperazino-1,8-naphthalimide. The chemosensor showed an about 17-fold increase in fluorescence quantum yield
Tridentate carbene CCC and CNC pincer palladium (II) complexes: structure, fluxionality, and catalytic activity.
Grundemann S, et al.
Organometallics, 20(25), 5485-5488 (2001)
V Balachandran et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 97, 1023-1032 (2012-08-29)
The FT-IR and FT-Raman spectra of 2,6-Bis(chloromethyl)pyridine (BCMP) have been recorded in the regions 4000-400 cm(-1) and 3500-100 cm(-1), respectively. The total energy calculations of BCMP were tried for the possible rotational isomers. The molecular structure, geometry optimization, vibrational frequencies
An efficient synthesis of 2,6-bis(chloromethyl)pyridine and a [5.5](2,6) pyridinophane disulfite.
Rezzonico B and Grignon-Dubois M.
J. Chem. Res. Synop., 4, 142-143 (1994)
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.