Alle Fotos(1)
Wichtige Dokumente
392510
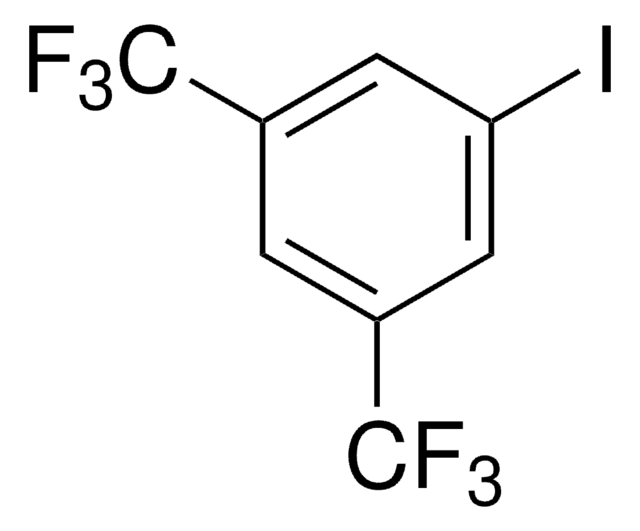
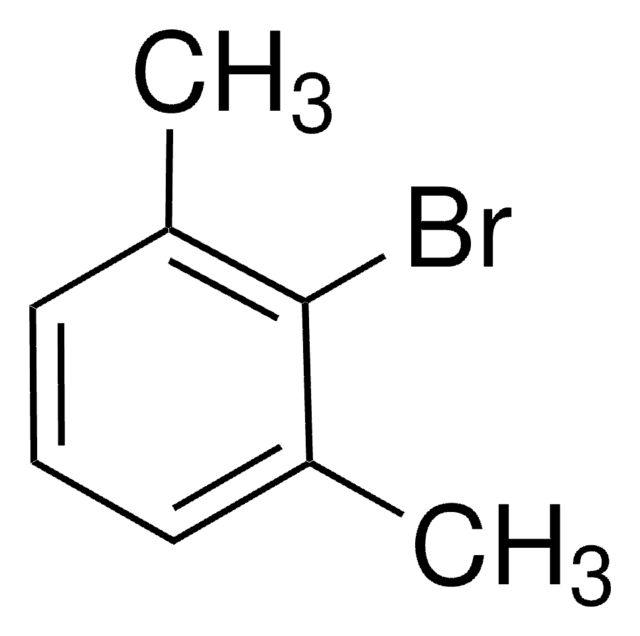
5-Iod-m-xylol
99%
Synonym(e):
1-Iod-3,5-dimethylbenzol
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Lineare Formel:
(CH3)2C6H3I
CAS-Nummer:
Molekulargewicht:
232.06
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Assay
99%
Form
liquid
Brechungsindex
n20/D 1.594 (lit.)
bp
92-94 °C/3 mmHg (lit.)
Dichte
1.608 g/mL at 25 °C (lit.)
Funktionelle Gruppe
iodo
SMILES String
Cc1cc(C)cc(I)c1
InChI
1S/C8H9I/c1-6-3-7(2)5-8(9)4-6/h3-5H,1-2H3
InChIKey
ZLMKEENUYIUKKC-UHFFFAOYSA-N
Verwandte Kategorien
Allgemeine Beschreibung
1-Iodo-3,5-dimethylbenzene (5-Iodo-m-xylene) is an aryl halide. It can be obtained from 5-bromo-m-xylene, via copper-catalyzed halogen exchange reaction, in the presence of NaI or KI in n-BuOH or DMF (solvents). It undergoes reaction with phenol in the presence of CuFe2O4 nano powder as a recyclable catalyst to afford 1,3-dimethyl-5-phenoxybenzene.
Anwendung
1-Iodo-3,5-dimethylbenzene (5-iodo-m-xylene) is suitable for use in the synthesis of N-(3,5-xylyl)-N-ethylaniline, an arylamine.
It may be used in the following studies:
It may be used in the following studies:
- α-Arylation of ketones.
- Copper-catalyzed N-arylation of imidazoles.
- Cyanation of 5-iodo-m-xylene to form 3,5-dimethylbenzonitrile.
- Synthesis of 1,3-Dimethyl-5-phenoxybenzene by nano-CuFe2O4 catalyzed C-O cross-coupling with phenol.
- CuBr-catalyzed amination of 1-iodo-3,5-dimethylbenzene to form N-Allyl-3,5-dimethylbenzenamine.
- Copper-catalyzed C-S bond-formation between 5-iodo-m-xylene and thiophenol.
- As a starting material in the synthesis of biphenyl-3,3′,5,5′-tetracarboxylic acid.
- Radical bromination of 5-iodo-m-xylene by N-bromosuccinimide to form 1,3-bis(bromomethyl)-5-iodobenzene.
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
10 - Combustible liquids
WGK
WGK 3
Flammpunkt (°F)
235.4 °F - closed cup
Flammpunkt (°C)
113 °C - closed cup
Persönliche Schutzausrüstung
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Anouk S Lubbe et al.
The Journal of organic chemistry, 76(21), 8599-8610 (2011-09-21)
A study is presented on the control of rotary motion of an appending rotor unit in a light-driven molecular motor. Two new light driven molecular motors were synthesized that contain aryl groups connected to the stereogenic centers. The aryl groups
Fuk Yee Kwong et al.
Organic letters, 4(20), 3517-3520 (2002-09-27)
An efficient copper-catalyzed carbon-sulfur bond formation reaction was developed. This method is particularly noteworthy given its experimental simplicity, high generality, and exceptional level of functional group toleration and the low cost of the catalyst system. [reaction: see text]
On the synthesis of heterocyclic dendrons.
Diez-Barra E, et al.
ARKIVOC (Gainesville, FL, United States), 2002(5), 17-25 (2002)
Recyclable and reusable nano-CuFe2O4 catalyzed CO cross-coupling.
Avudoddi V, et al.
European Journal of Chemistry, 3(3), 298-304 (2012)
Recyclable and reusable nano-CuFe2O4 catalyzed CO cross-coupling.
Avudoddi V, et al.
European Journal of Organic Chemistry, 3(3), 298-304 (2012)
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.