Alle Fotos(1)
Wichtige Dokumente
381055
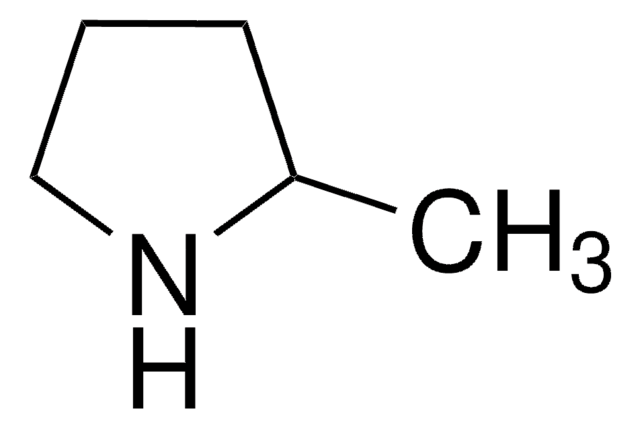
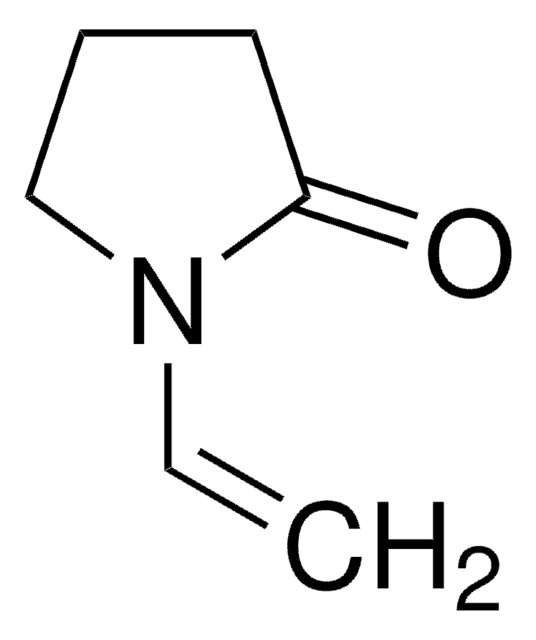
2-Methyl-1-pyrrolin
95%
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C5H9N
CAS-Nummer:
Molekulargewicht:
83.13
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
95%
Brechungsindex
n20/D 1.444 (lit.)
bp
104-105 °C (lit.)
Dichte
0.878 g/mL at 25 °C (lit.)
SMILES String
CC1=NCCC1
InChI
1S/C5H9N/c1-5-3-2-4-6-5/h2-4H2,1H3
InChIKey
CTSZPNIMMLSKDV-UHFFFAOYSA-N
Allgemeine Beschreibung
2-Methyl-1-pyrroline, a monocyclic imine, is a pyrroline derivative. It is a five-membered heterocyclic compound having various biological and pharmacological applications. It is formed during the Rh(I) complexes (containing N,N-donor ligands and N,P-donor ligand) immobilized on glassy carbon electrode surfaces catalyzed intramolecular hydroamination of 4-pentyn-1-amine. It reacts with with 2-oxopropanal to afford acetyl-1-pyrroline (AP).
Anwendung
2-Methyl-1-pyrroline (2-MPN) may be used in the enantioselective enzymatic synthesis of (R)- and (S)-2-methylpyrroline in the presence of whole-cells catalysts isolated from Strptomyces sp. strains GF3587 and GF3546 respectively.
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
3 - Flammable liquids
WGK
WGK 3
Flammpunkt (°F)
51.8 °F - closed cup
Flammpunkt (°C)
11 °C - closed cup
Persönliche Schutzausrüstung
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
2-Oxopropanal, hydroxy-2-propanone, and 1-pyrroline important intermediates in the generation of the roast-smelling food flavor compounds 2-acetyl-1-pyrroline and 2-acetyltetrahydropyridine.
Hofmann T and Schieberle P.
Journal of Agricultural and Food Chemistry, 46(6), 2270-2277 (1998)
María Rodríguez-Mata et al.
Chembiochem : a European journal of chemical biology, 14(11), 1372-1379 (2013-07-03)
NADPH-dependent oxidoreductase Q1EQE0 from Streptomyces kanamyceticus catalyzes the asymmetric reduction of the prochiral monocyclic imine 2-methyl-1-pyrroline to the chiral amine (R)-2-methylpyrrolidine with >99% ee, and is thus of interest as a potential biocatalyst for the production of optically active amines.
Andrey A Tregubov et al.
Journal of the American Chemical Society, 135(44), 16429-16437 (2013-10-04)
A series of N,N-donor ligands (bis(pyrazol-1-yl)methane (bpm), bis(N-methylimidazol-2-yl)methane (bim), 1-(phenylmethyl)-4-(1H-pyrazol-1-yl methyl)-1H-1,2,3-triazole (PyT)), and one N,P-donor ligand precursor (1-(3,5-dimethylpyrazol-1-yl)(2-bromoethane) (dmPyBr)) were synthesized and functionalized with aniline. Diazotization of the aniline into an aryl diazonium, using nitrous acid in aqueous conditions, was
M Bertoldi et al.
The Biochemical journal, 342 Pt 3, 509-512 (1999-09-08)
Ornithine decarboxylase (ODC) from Lactobacillus 30a catalyses the cleavage of alpha-methylornithine into ammonia and 2-methyl-1-pyrroline; glutamate decarboxylase (GAD) from Escherichia coli catalyses the cleavage of alpha-methylglutamate into ammonia and laevulinic acid. In our analyses, 2-methyl-1-pyrroline and laevulinic acid were identified
Koichi Mitsukura et al.
Bioscience, biotechnology, and biochemistry, 75(9), 1778-1782 (2011-09-08)
The (R)-imine reductase (RIR) of Streptomyces sp. GF3587 was purified and characterized. It was found to be a NADPH-dependent enzyme, and was found to be a homodimer consisting of 32 kDa subunits. Enzymatic reduction of 10 mM 2-methyl-1-pyrroline (2-MPN) resulted
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.