Alle Fotos(2)
Wichtige Dokumente
311073
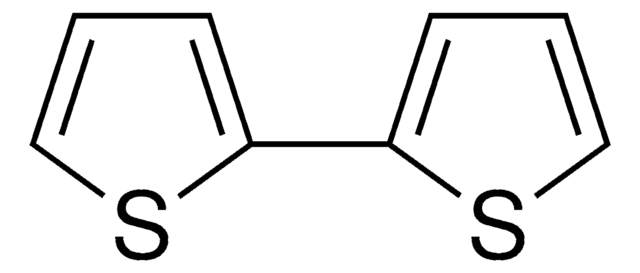
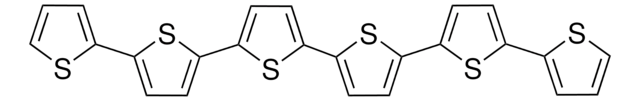
2,2′:5′,2′′-Terthiophen
99%
Synonym(e):
α-Terthienyl, 2,5-Di-(2-thienyl)-thiophen
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(2)
About This Item
Empirische Formel (Hill-System):
C12H8S3
CAS-Nummer:
Molekulargewicht:
248.39
Beilstein:
178604
MDL-Nummer:
UNSPSC-Code:
12352103
PubChem Substanz-ID:
NACRES:
NA.23
Empfohlene Produkte
Assay
99%
mp (Schmelzpunkt)
93-95 °C (lit.)
SMILES String
c1csc(c1)-c2ccc(s2)-c3cccs3
InChI
1S/C12H8S3/c1-3-9(13-7-1)11-5-6-12(15-11)10-4-2-8-14-10/h1-8H
InChIKey
KXSFECAJUBPPFE-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
2,2′:5′,2′′-Terthiophene (3T) is a tri-thiophene based low band conductive polymer that is prepared by reacting 2,5-dibromothiophene and thienylmagnesium bromide in the presence of nickel catalyst.
2,2′:5′,2′′-Terthiophene (TTh) may be prepared by nickel catalysed coupling reaction of grignard′s reagent derived from 2-bromothiophene and magnesium. It generates singlet oxygen. In nature, it is found in the floral extract of Tagetes minuta and Echinops grijisii. It is known to be toxic to mosquitoes. It also exihibits antifungal activity.
Anwendung
3T can be combined with 3,4-ethylenedioxythiophene (EDOT) in a tetrabutylammonium perchlorate solution for use as an electrochromic copolymer for a wide range of applications like photovoltaics and polymer light emitting diodes (LEDs). It can also be used to form metal-organic based thin films with metals like aluminum, silver, and calcium which can potentially be used for optoelectronics based applications.
Electrochemical copolymerization of carbazole and TTh in sodium perchlorate/acetonitrile was reported. Electrochromic copolymer based on TTh and 3, 4-ethylenedioxythiophene has been reported. TTh acts as a monomer precursor for polythiophene and as a dopant for polycarbonate. It may function as a photosensitizer.
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Synthesis and characterization of an electrochromic copolymer based on 2, 2′ : 5′ , 2″ -terthiophene and 3, 4-ethylenedioxythiophene
Ahmed MS, et al.
Applied Nanoscience, 2(2), 133-141 (2012)
Man Jae Park et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 18(7), 2056-2062 (2012-01-18)
Excess-electron transfer (EET) in DNA has attracted wide attention owing to its close relation to DNA repair and nanowires. To clarify the dynamics of EET in DNA, a photosensitizing electron donor that can donate an excess electron to a variety
Journal of Non-Crystalline Solids, 164, 1263-1263 (1993)
Hui-Bog Noh et al.
Biomaterials, 33(9), 2600-2607 (2012-01-03)
A highly sensitive in vivo biosensor for glutathione disulfide (GSSG) is developed using covalently immobilized-glutathione reductase (GR) and -β-nicotinamide adenine dinucleotide phosphate (NADPH) on gold nanoparticles deposited on poly[2,2':5',2″-terthiophene-3'-(p-benzoic acid)] (polyTTBA). The fabricated biosensor was characterized with SEM, TEM, XPS
Synthesis and characterization of an electrochromic copolymer based on 2,2':5',"-terthiophene and 3,4-ethylenedioxythiophene
Ahmed MS, et al.
Applied Nanoscience, 2, 133-141 (2012)
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.


![Benzo[1,2-b:4,5-b′]dithiophene-4,8-dione 97%](/deepweb/assets/sigmaaldrich/product/structures/418/544/b7faac0b-ad09-4b42-a9fa-aeb38017a39e/640/b7faac0b-ad09-4b42-a9fa-aeb38017a39e.png)