Alle Fotos(1)
Wichtige Dokumente
247847
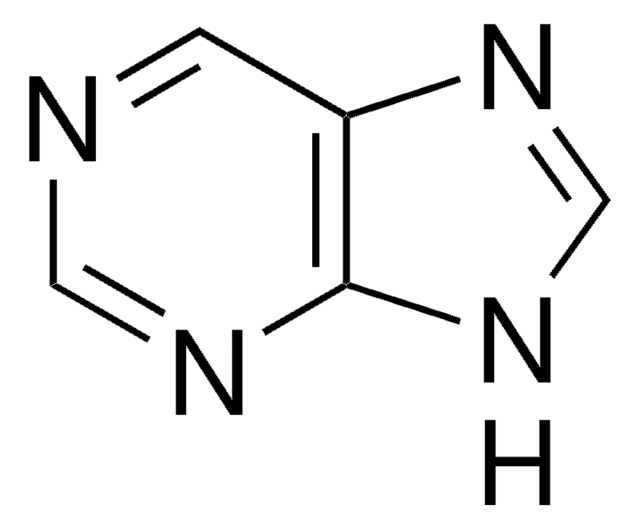
2,6-Diaminopurin
98%
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C5H6N6
CAS-Nummer:
Molekulargewicht:
150.14
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12162002
PubChem Substanz-ID:
NACRES:
NA.23
Empfohlene Produkte
Qualitätsniveau
Assay
98%
mp (Schmelzpunkt)
>300 °C (lit.)
SMILES String
Nc1nc(N)c2nc[nH]c2n1
InChI
1S/C5H6N6/c6-3-2-4(9-1-8-2)11-5(7)10-3/h1H,(H5,6,7,8,9,10,11)
InChIKey
MSSXOMSJDRHRMC-UHFFFAOYSA-N
Verwandte Kategorien
Signalwort
Warning
Gefahreneinstufungen
Acute Tox. 4 Oral - Carc. 2 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Søren Lindemose et al.
Nucleic acids research, 36(14), 4797-4807 (2008-07-26)
The DNA interaction of the Escherichia coli cyclic AMP receptor protein (CRP) represents a typical example of a dual recognition mechanism exhibiting both direct and indirect readout. We have dissected the direct and indirect components of DNA recognition by CRP
V Krishnakumar et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 69(1), 8-17 (2007-05-01)
FT-IR and FT-Raman spectra of 2,6-diamino purine (DAP) and 6-methoxy purine (MP) have been recorded in the regions of 4000-400cm(-1) and 3500-100cm(-1), respectively. The spectra were interpreted with the aid of normal coordinate analysis following full structure optimizations and force
Miguel A Galindo et al.
Inorganic chemistry, 48(23), 11085-11091 (2009-10-28)
Alkyldiamine-tethered derivatives of 2,6-diaminopurine, ethylenediamine-N9-propyl-2,6-diaminopurine, L1, and ethylenediamine-N9-ethyl-2,6-diaminopurine, L2, react with Pd(II) to give N3-coordinated complexes. However, the exact nature of the resulting complex is dependent on the reaction conditions. With PdCl(2)(MeCN)(2) in MeCN/H(2)O the expected [PdCl(N3-2,6-DAP-alkyl-en)](+) complex, 1, is
Elizabeth Mburu et al.
The journal of physical chemistry. A, 112(48), 12485-12491 (2008-11-07)
Several excited singlet electronic states of purine nucleobases and related derivatives have been calculated using high-level multireference perturbation theory methods. Purine derivatives with one or two amino or carbonyl groups substituted at positions C(2) and/or C(6) of the purine ring
Kiyohiko Kawai et al.
Journal of the American Chemical Society, 132(2), 627-630 (2009-12-18)
A positive charge migrates along DNA mainly via a series of short-range charge transfer (CT) processes between G-C base pairs, which have relatively high HOMO levels. As such, the CT efficiency sharply decreases with the insertion of A-T base pairs
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.