Wichtige Dokumente
229601
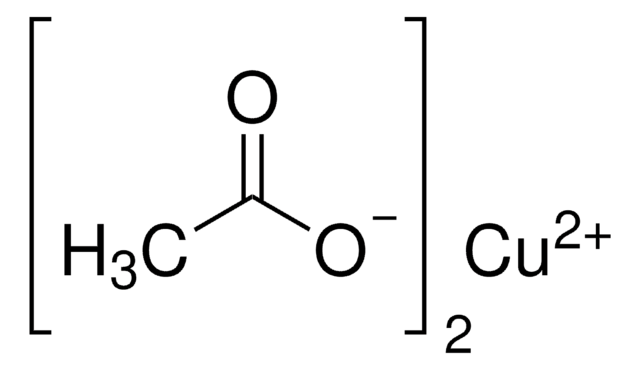
Kupfer(II)-acetat Monohydrat
99.99% trace metals basis
Synonym(e):
Cupric acetate monohydrate
About This Item
Empfohlene Produkte
Dampfdichte
6.8 (vs air)
Qualitätsniveau
Assay
99.99% trace metals basis
Form
powder or crystals
Eignung der Reaktion
core: copper
Grünere Alternativprodukt-Eigenschaften
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Grünere Alternativprodukt-Kategorie
SMILES String
O.CC(=O)O[Cu]OC(C)=O
InChI
1S/2C2H4O2.Cu.H2O/c2*1-2(3)4;;/h2*1H3,(H,3,4);;1H2/q;;+2;/p-2
InChIKey
NWFNSTOSIVLCJA-UHFFFAOYSA-L
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Allgemeine Beschreibung
Anwendung
- To synthesize CuSbS2 nanoplates and a CuSbS2-Cu3SbS4 nanocomposite via hot injection method. CuSbS2 can be used as an absorber material in solar cells due to its favorable optical properties and direct band gap. The CuSbS2-Cu3SbS4 nanocomposite exhibits promising super capacitive properties, making it suitable for energy storage applications.
- To synthesize copper oxide nanoparticles (CuO NPs) using a green synthesis method involving psidium guajava leaf extract as both a reducing and capping agent. The CuO NPs exhibit excellent photocatalytic activity for degrading industrial dyes, such as Nile Blue (NB) and Reactive Yellow 160 (RY160). These nanoparticles can be used for purifying water resources contaminated with industrial dyes.
- As a copper precursor in synthesizing CuO semiconducting thin films via jet nebulizer spray pyrolysis technique, for P–N diode application. These CuO films find applications in supercapacitors, sensors, solar cells, photocatalysis and electrochromic devices.
Leistungsmerkmale und Vorteile
- It is soluble in water makes a perfect precursor for the synthesis of new materials by sol-gel method
- With high purity of 99.99% (<150 ppm) and low heavy metals, it is ideal for Ir-catalyzed intramolecular C-H amination and the synthesis of carbinol.
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 2 - Eye Dam. 1 - Skin Corr. 1B
Lagerklassenschlüssel
8B - Non-combustible corrosive hazardous materials
WGK
WGK 3
Flammpunkt (°F)
does not flash
Flammpunkt (°C)
does not flash
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.