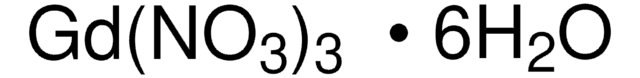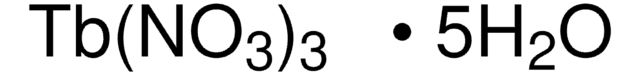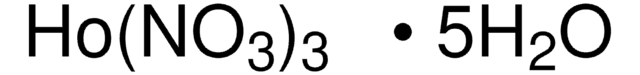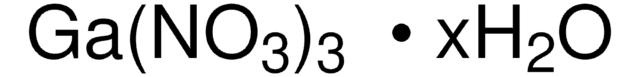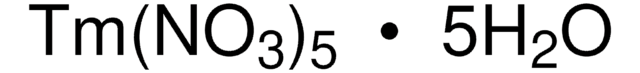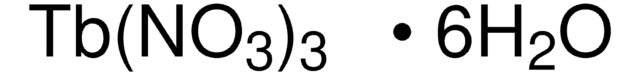211591
Gadolinium(III)-nitrat Hexahydrat
crystals and lumps, 99.9% trace metals basis
Synonym(e):
Gadolinium hexahydrate trinitrate
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
99.9% trace metals basis
Form
crystals and lumps
Eignung der Reaktion
reagent type: catalyst
core: gadolinium
Verunreinigungen
≤1500.0 ppm Trace Rare Earth Analysis
mp (Schmelzpunkt)
91 °C (lit.)
SMILES String
[Gd+3].[H]O[H].[H]O[H].[H]O[H].[H]O[H].[H]O[H].[H]O[H].[O-][N+]([O-])=O.[O-][N+]([O-])=O.[O-][N+]([O-])=O
InChI
1S/Gd.3NO3.6H2O/c;3*2-1(3)4;;;;;;/h;;;;6*1H2/q+3;3*-1;;;;;;
InChIKey
XWFVFZQEDMDSET-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
- As a precursor to synthesize Gadolinium oxide (Gd2O3) nanoparticles for various applications such as photocatalysis and biomedical imaging.
- As a dopant to prepare ZnO nanoparticles with enhanced room temperature ferromagnetism.
- To prepare Ceria (CeO2)-based ceramics doped electrolytes for intermediate temperature solid oxide fuel cells (IT-SOFCs) .
- As a raw material for the synthesis of Eu3+-Activated KGd2F7 red-emitting nanoparticles for white light-emitting diode.
- In addition,it is also reported to producegreen light emission of Er3+-activated single-phased GdAlO3 phosphors for lighting applications.
Sonstige Hinweise
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Ox. Sol. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
5.1B - Oxidizing hazardous materials
WGK
WGK 2
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
The rare earth elements impact nearly everyone in the world. All of the people living in advanced technological countries and almost all those living in third world countries utilize the rare earths in their everyday living—the car that one drives (gasoline is refined from oil using rare earth catalysts and catalytic converters reduce the polluting emissions from the automotive exhaust), watching the news on TV (the red and green colors in TV screens), the telephones and computers we use to communicate (the permanent magnets in speakers and disc drives), just to name a few examples.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.