Alle Fotos(1)
Wichtige Dokumente
123617
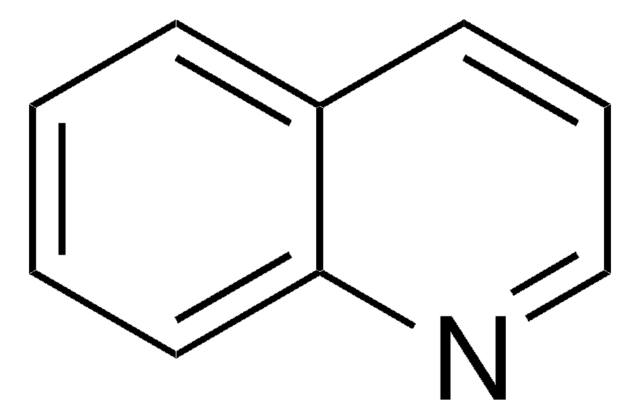
Benzo[h]chinolin
97%
Synonym(e):
α-Naphthochinolin, 7,8-Benzochinolin
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C13H9N
CAS-Nummer:
Molekulargewicht:
179.22
Beilstein:
120249
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
97%
Form
solid
bp
338 °C/719 mmHg (lit.)
mp (Schmelzpunkt)
48-50 °C (lit.)
SMILES String
c1ccc2c(c1)ccc3cccnc23
InChI
1S/C13H9N/c1-2-6-12-10(4-1)7-8-11-5-3-9-14-13(11)12/h1-9H
InChIKey
WZJYKHNJTSNBHV-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Anwendung
Benzo[h]quinoline was used to study the mutagenic activities of benzo[f]quinoline, benzo[h]quinolone and a number of their derivatives in strain TA 100 of Salmonella typhimurium. It was used in determination of nitrogen-containing polynuclear aromatic hydrocarbons in the gaseous products of the thermal degradation of polymers by HPLC- fluorescence detection. It was used as starting reagent for the synthesis of osmium and ruthenium complexes containing an N-heterocyclic carbene ligand.
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
235.4 °F - closed cup
Flammpunkt (°C)
113 °C - closed cup
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
E J LaVoie et al.
Carcinogenesis, 4(9), 1133-1138 (1983-09-01)
Benzo[f]quinoline and benzo[h]quinoline are widespread environmental pollutants which have been found to be mutagenic. The metabolism of benzo[f]quinoline and benzo[h]quinoline was investigated using a liver homogenate from Aroclor-pretreated rats. The metabolites of benzo[f]quinoline which were identified were 7,8-dihydroxy-7,8-dihydrobenzo[f]quinoline, 9,10-dihydroxy-9,10-dihydrobenzo[f]quinoline, 7-hydroxybenzo[f]quinoline
Marko Weimar et al.
Organic & biomolecular chemistry, 11(1), 31-34 (2012-10-17)
The challenging coupling of 10-halobenzo[h]quinolines with ortho-substituted aryl boronic acids has been achieved using Pd(OAc)(2)/P(O)Ph(3) as the catalytic system. High yields were obtained for diversely functionalised substrates under mild reaction conditions.
E J LaVoie et al.
Japanese journal of cancer research : Gann, 78(2), 139-143 (1987-02-01)
The environmental occurrence and mutagenic activity of quinoline and benzoquinolines are well-documented. In this study, the relative carcinogenic activities of quinoline, benzo[f]quinoline, benzo[h]quinoline, and phenanthridine were evaluated in newborn mice. Mice were injected intraperitoneally on the first, eighth, and fifteenth
Hanumantharao Paritala et al.
Bioorganic & medicinal chemistry letters, 19(6), 1584-1587 (2009-02-27)
G-quadruplexes are unusual structures formed from guanine-rich sequences of nucleic acids. G-quadruplexes have been postulated to play important roles in a number of biological systems including gene regulation and the inhibition of enzyme function. Recently, our laboratory reported on the
John B Sutherland et al.
Applied microbiology and biotechnology, 67(3), 405-411 (2005-04-28)
Cultures of Umbelopsis ramanniana (=Mucor ramannianus) were grown in fluid Sabouraud medium for 3 days, dosed with 0.23 mM benzo[f]quinoline, benzo[h]quinoline, or phenanthridine (benzo[c]quinoline), and incubated for another 18 days. Cultures were extracted and metabolites (66-75% of the UV absorbance)
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.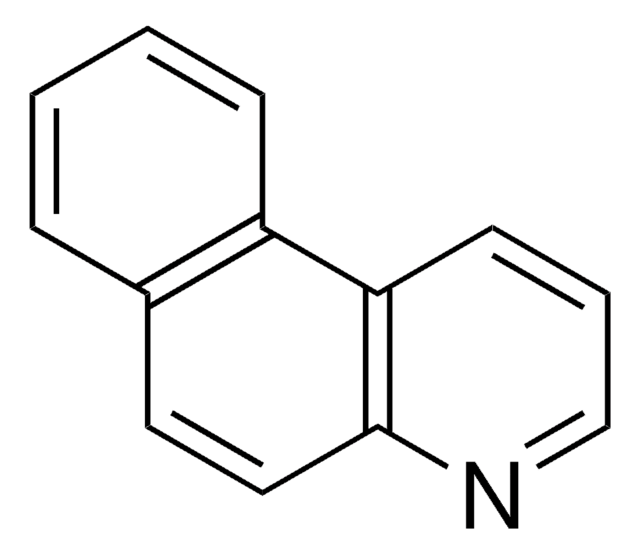




![Dibenz[c,h]acridin BCR®, certified reference material](/deepweb/assets/sigmaaldrich/product/structures/364/643/698df9fb-5b7d-467a-b47e-c8318e2ed298/640/698df9fb-5b7d-467a-b47e-c8318e2ed298.png)
![Benz[g]isochinolin-5,10-dion 99%](/deepweb/assets/sigmaaldrich/product/structures/484/029/288c4a9d-19c2-4b51-82c1-f43b50ea05b0/640/288c4a9d-19c2-4b51-82c1-f43b50ea05b0.png)