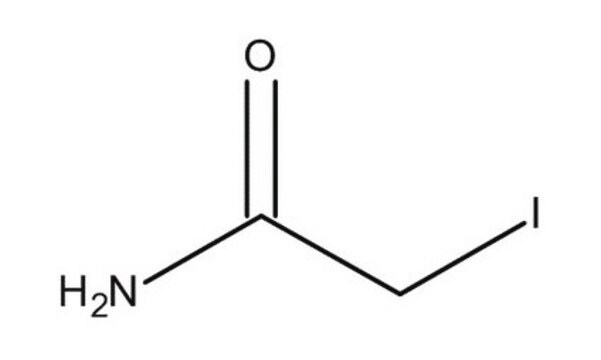
I6125
Iodoacetamide
≥99% (NMR), crystalline
Synonym(s):
α-Iodoacetamide, 2-Iodoacetamide
About This Item
Recommended Products
biological source
synthetic (organic)
Quality Level
Assay
≥99% (NMR)
form
crystalline
mp
92-95 °C (lit.)
solubility
H2O: soluble 0.5 M, clear, colorless
storage temp.
2-8°C
SMILES string
NC(=O)CI
InChI
1S/C2H4INO/c3-1-2(4)5/h1H2,(H2,4,5)
InChI key
PGLTVOMIXTUURA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- A Mass Spectrometry Strategy for Protein Quantification Based on the Differential Alkylation of Cysteines Using Iodoacetamide and Acrylamide.: This study presents a novel mass spectrometry method for quantifying proteins by differentially alkylating cysteines with iodoacetamide and acrylamide, enhancing the accuracy of protein quantification in complex samples. (Virág et al., 2024).
- Laminarin ameliorates iodoacetamide-induced functional dyspepsia via modulation of 5-HT(3) receptors and the gut microbiota.: Research demonstrates that laminarin can mitigate functional dyspepsia induced by iodoacetamide through modulating 5-HT(3) receptors and altering gut microbiota composition, offering potential therapeutic benefits. (Liu et al., 2024).
- Redox proteomics in melanoma cells: An optimized protocol.: This paper describes an optimized redox proteomics protocol using iodoacetamide, which facilitates the identification of redox-sensitive proteins in melanoma cells, aiding in the understanding of redox regulation in cancer. (Cunha et al., 2024).
- Molecular targets of cisplatin in HeLa cells explored through competitive activity-based protein profiling strategy.: Utilizing iodoacetamide in competitive activity-based protein profiling, this study identifies molecular targets of cisplatin in HeLa cells, providing insights into the drug′s mechanisms. (Chen et al., 2024).
- Development and Comparison of 4-Thiouridine to Cytidine Base Conversion Reaction.: This research compares base conversion reactions involving 4-thiouridine and cytidine, with iodoacetamide playing a crucial role in the reaction mechanism, contributing to advancements in nucleotide chemistry. (Ohashi et al., 2024).
Biochem/physiol Actions
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Aquatic Chronic 4 - Resp. Sens. 1 - Skin Sens. 1
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service