D3415
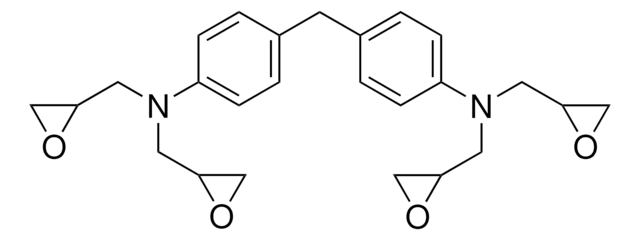
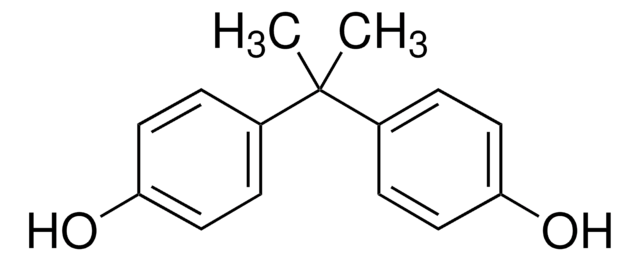
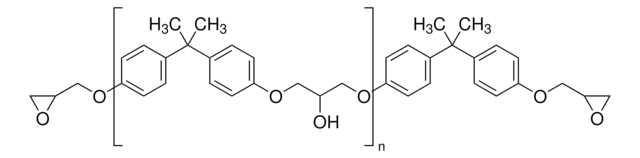
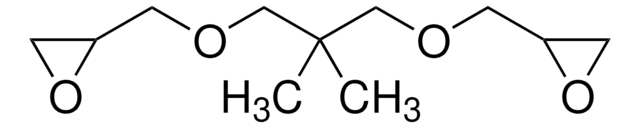
Bisphenol A diglycidyl ether
viscous liquid, PPARγ inhibitor
Synonym(s):
BADGE, DGEBA, NSC 5022, 2,2-Bis[4-(glycidyloxy)phenyl]propane, 4,4′-Isopropylidenediphenol diglycidyl ether, D.E.R.™ 332
About This Item
Recommended Products
product name
Bisphenol A diglycidyl ether,
form
viscous liquid
Quality Level
composition
Epoxide equivalent weight, 172-176
density
1.16 g/mL at 25 °C (lit.)
SMILES string
CC(C)(c1ccc(OCC2CO2)cc1)c3ccc(OCC4CO4)cc3
InChI
1S/C21H24O4/c1-21(2,15-3-7-17(8-4-15)22-11-19-13-24-19)16-5-9-18(10-6-16)23-12-20-14-25-20/h3-10,19-20H,11-14H2,1-2H3
InChI key
LCFVJGUPQDGYKZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- to study its effects on propiomelanocortin (POMC):enhanced green fluorescent protein (EGFP) expression in our transgenic fish
- to study the curing behavior, kinetics of epoxy, polyamidoamine systems and the influence of the incorporation of Fe3O4 magnetic nanoparticles (MNPs)
- as a thermosetting resin
- to determine the optimum composition of the epoxy-polysiloxane blend
- for the preparation of transparent epoxy-based nanocomposite coatings
- for the preparation, coating and curing of epoxy-graphene (E/G)
Biochem/physiol Actions
Features and Benefits
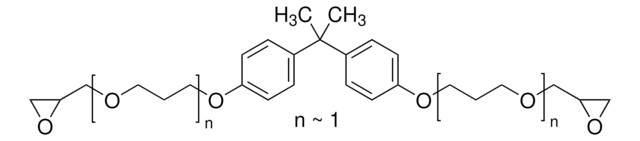
Linkage
Legal Information
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2 - Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
507.2 - 514.4 °F - closed cup
Flash Point(C)
264 - 268 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
SupelMIP SPE-Bisphenol A gave high recovery for baseline resolution of five related compounds with LC-Fluorescence detection.
Rapid potency testing of marijuana-infused edibles using LC/MS on a biphenyl stationary phase detected eleven cannabinoids.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service