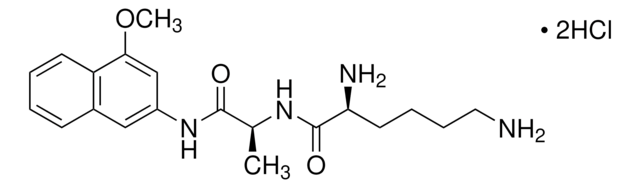
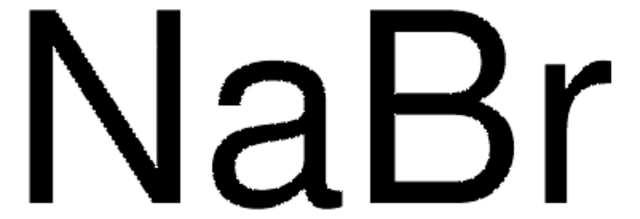All Photos(4)
About This Item
Linear Formula:
CH3COOK
CAS Number:
Molecular Weight:
98.14
Beilstein:
3595449
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.21
grade:
reagent
form:
powder or crystals
Recommended Products
grade
reagent
vapor pressure
<0.0000001 hPa ( 25 °C)
product line
ReagentPlus®
Assay
≥99.0%
form
powder or crystals
pH
7-9 (25 °C, 98.2 g/L)
density
1.57 g/cm3 at 25 °C (lit.)
SMILES string
[K+].CC([O-])=O
InChI
1S/C2H4O2.K/c1-2(3)4;/h1H3,(H,3,4);/q;+1/p-1
InChI key
SCVFZCLFOSHCOH-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
General description
Potassium acetate is an osmotic agent that can be prepared by the reaction between acetic acid and potassium hydroxide. It has replaced urea and glycol for deicing process. Its potential as a substitute to calcium chloride for osmotic distillation has been investigated. It intercalates with the hydroxyl groups on the kaolinite surface leading to surface modification. It acts as a catalyst that accelerates the acetylation of wood at low temperature.
Application
Potassium acetate has been used during granule bound starch synthase (GBSS) activity assay. It may be used as an activator to produce waste tea activated carbon (WTAC).
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Potassium acetate-catalyzed acetylation of wood: reaction rates at low temperatures.
Obataya E and Minato K.
Wood Science and Technology, 43(5-6), 405-413 (2009)
M M Shah et al.
Applied biochemistry and biotechnology, 63-65, 423-433 (1997-04-01)
Potassium acetate is currently made by reacting petroleum-based acetic acid with potassium hydroxide. An alternate process, anaerobic fermentation of dextrose with Clostridium thermoaceticum, could be used and could possibly be cheaper. Growth characteristics and productivity of the fermentation were optimized
Purification of starch granules from Arabidopsis leaves and determination of granule-bound starch synthase activity.
Albi T, et al.
Bio-protocol, 4(23), e1316-e1316 (2013)
Preparation of waste tea activated carbon using potassium acetate as an activating agent for adsorption of Acid Blue 25 dye.
Auta M and Hameed BH.
Chemical Engineering Journal, 171(2), 502-509 (2011)
Modification of kaolinite surfaces through intercalation with potassium acetate, II.
Frost RL, et al.
Journal of Colloid and Interface Science, 214(1), 109-117 (1999)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service