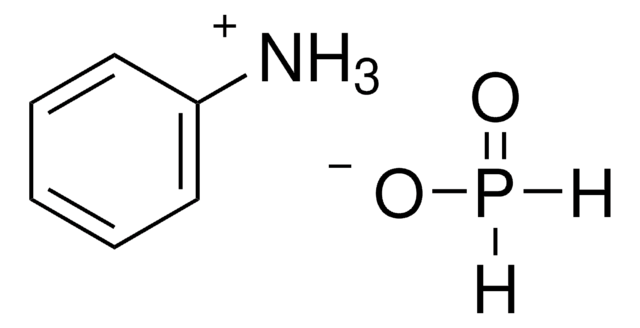
214906
Hypophosphorous acid solution
50 wt. % in H2O
Synonym(s):
Phosphinic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
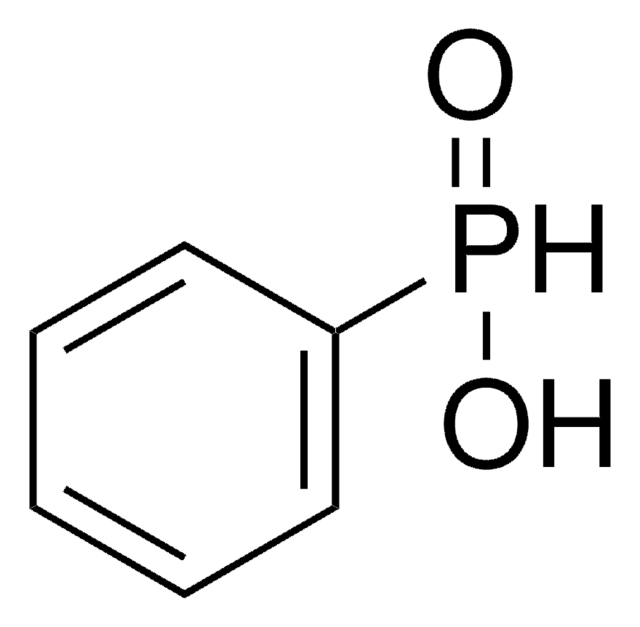
Linear Formula:
H3PO2
CAS Number:
Molecular Weight:
66.00
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
vapor pressure
<17 mmHg ( 20 °C)
Quality Level
form
liquid
concentration
48-52% in NaOH (titration)
50 wt. % in H2O
pH
1 (20 °C, 500 g/L)
density
1.206 g/mL at 25 °C
SMILES string
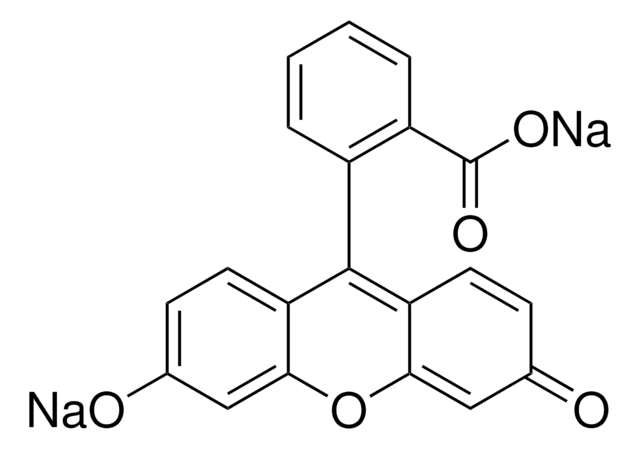
O[PH2]=O
InChI
1S/H3O2P/c1-3-2/h3H2,(H,1,2)
InChI key
ACVYVLVWPXVTIT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
H3PO2, a monobasic oxyacid, is also referred to as phosphinic acid. H3PO2 participates as a reducing agent for the conversion of aromatic and aliphatic diselenides to the corresponding selenols.
It undergoes oxidation in the presence of Ce(IV) and a strong acid to afford cerium(IV) hypophosphite complex ions.
It undergoes oxidation in the presence of Ce(IV) and a strong acid to afford cerium(IV) hypophosphite complex ions.
Application
- Synthesis of Aminobisphosphinates through a Cascade Reaction between Hypophosphorous Acid and Bis(trimethylsilyl)imidates Mediated by ZnI(2).: This research presents a novel method for synthesizing aminobisphosphinates, utilizing hypophosphorous acid in a zinc iodide-mediated cascade reaction, which could be relevant in the development of new chemical entities in medicinal chemistry (Ayadi et al., 2023).
- Facile Synthesis of Liquid Crystal Dimers Bridged with a Phosphonic Group.: This study explores the use of hypophosphorous acid in the synthesis of phosphonic group-bridged liquid crystal dimers, offering potential advancements in materials science and display technologies (Wang et al., 2022).
- Oxidation of Hypophosphorous Acid by a Ruthenium(VI) Nitrido Complex in Aqueous Acidic Solution. Evidence for a Proton-Coupled N-Atom Transfer Mechanism.: Investigates the mechanistic details of hypophosphorous acid oxidation by a ruthenium complex, shedding light on reaction pathways important in catalysis and synthesis (Li et al., 2022).
- Solution-processed Ge(ii)-based chalcogenide thin films with tunable bandgaps for photovoltaics.: Discusses the application of hypophosphorous acid in the development of germanium-based chalcogenide thin films, contributing to advancements in photovoltaic materials (Hu et al., 2022).
- One-Pot Synthesis and in Silico Molecular Docking Studies of Arylselanyl Hydrazides as Potential Antituberculosis Agents.: Utilizes hypophosphorous acid in the one-pot synthesis of arylselanyl hydrazides, evaluated for their potential as antituberculosis agents through molecular docking studies (Borges et al., 2022).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Met. Corr. 1 - Skin Corr. 1B
Storage Class Code
8B - Non-combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The Kinetics and Mechanism of the Oxidation of Hypophosphorous Acid by Cerium (IV) in Perchloric Acid Solution.
Carroll RL and Thomas LB.
Journal of the American Chemical Society, 88(7), 1376-1381 (1996)
Hypophosphorous acid mediated dehalogenation in water.
Jang DO.
Tetrahedron Letters, 37(30), 5367-5368 (1996)
Jayesh Cherusseri et al.
Nanoscale, 12(18), 10072-10081 (2020-04-30)
We present a simple and facile method to synthesize nanoplatelets of 2D Ruddlesden-Popper (RP) perovskites of the type (CH3(CH2)3NH3)2(CH3NH3)Pb2I7 where n = 2. The 2D RP nanoplatelets are synthesized from bulk 2D RP crystals via a reflux pre-treatment mediated-ultrasonication method.
Kai Wang et al.
ACS nano, 12(5), 4919-4929 (2018-04-24)
The robust material stability of the quasi-two-dimensional (quasi-2D) metal halide perovskites has opened the possibility for their usage instead of three-dimensional (3D) perovskites. Further, devices based on large area single crystal membranes have shown increasing promise for photoelectronic applications. However
Hypophosphorous Acid, a Novel Reagent for the Reduction of Diselenides and the Selenol-Catalyzed Reduction of Disulfides1,2.
Gunther WHH.
The Journal of Organic Chemistry, 31(4), 1202-1205 (1966)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service