E2503004
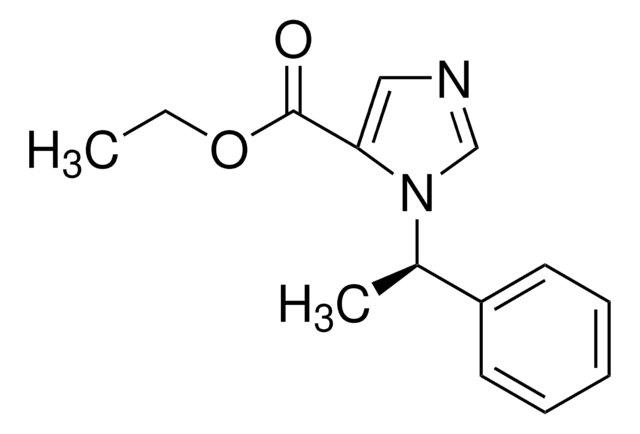
Etomidate impurity B
European Pharmacopoeia (EP) Reference Standard
Synonym(s):
Methyl 1-[(1RS)-1-phenylethyl]-1H-imidazole-5-carboxylate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C13H14N2O2
CAS Number:
Molecular Weight:
230.26
UNSPSC Code:
41116107
NACRES:
NA.24
Recommended Products
grade
pharmaceutical primary standard
API family
etomidate
manufacturer/tradename
EDQM
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
2-8°C
InChI
1S/C13H14N2O2/c1-10(11-6-4-3-5-7-11)15-9-14-8-12(15)13(16)17-2/h3-10H,1-2H3
InChI key
FHFZEKYDSVTYLL-UHFFFAOYSA-N
General description
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
Application
Etomidate impurity B EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
Packaging
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
Other Notes
Sales restrictions may apply.
related product
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Tina C Crosby et al.
Journal of aquatic animal health, 24(2), 73-80 (2012-07-31)
Our objectives were to determine whether sedation with metomidate hydrochloride (hereafter, "metomidate") during transportation of threespot gourami Trichogaster trichopterus would prevent an increase in blood glucose levels and improve fish marketability (i.e., based on appearance and behavior) in comparison with
Maria Erlandsson et al.
Nuclear medicine and biology, 36(4), 435-445 (2009-05-09)
Two- and one-step syntheses of (18)F-labelled analogues of metomidate, such as 2-[(18)F]fluoroethyl 1-[(1R)-1-phenylethyl]-1H-imidazole-5-carboxylate (1), 2-[(18)F]fluoroethyl 1-[(1R)-1-(4-chlorophenyl)ethyl]-1H-imidazole-5-carboxylate (2), 2-[(18)F]fluoroethyl 1-[(1R)-1-(4-bromophenyl)ethyl]-1H-imidazole-5-carboxylate (3), 3-[(18)F]fluoropropyl 1-[(1R)-1-(4-bromophenyl)ethyl]-1H-imidazole-5-carboxylate (4) and 3-[(18)F]fluoropropyl 1-[(1R)-1-phenylethyl]-1H-imidazole-5-carboxylate (5) are presented. Analogues 1-5 were prepared by a two-step reaction sequence that started
Kenneth B Davis et al.
Comparative biochemistry and physiology. Toxicology & pharmacology : CBP, 143(1), 134-139 (2006-02-10)
Channel catfish and sunshine bass were exposed to a low-water stress event and allowed to recover in fresh water or a solution of metomidate (dl-1-(1-phenylethyl)-5-(metoxycarbonyl) imidazole hydrochloride), which inhibits the synthesis of cortisol. Change in time of plasma cortisol was
M K Hansen et al.
Journal of veterinary pharmacology and therapeutics, 26(2), 95-103 (2003-04-02)
Metomidate was administered to halibut (Hippoglossus hippoglossus) and turbot (Scophthalmus maximus) intravenously at a dose of 3 mg/kg bodyweight, as a bath treatment at a dose of 9 mg/L water for 5 min to study the disposition of metomidate, and
Grayson A Doss et al.
Journal of zoo and wildlife medicine : official publication of the American Association of Zoo Veterinarians, 45(1), 53-59 (2014-04-10)
Metomidate hydrochloride is an imidazole-based, nonbarbiturate hypnotic drug primarily used as an immersion sedation and anesthetic agent in freshwater and marine finfish. To the authors' knowledge, there is no documentation in the literature of its use in amphibians. In this
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service