E-015
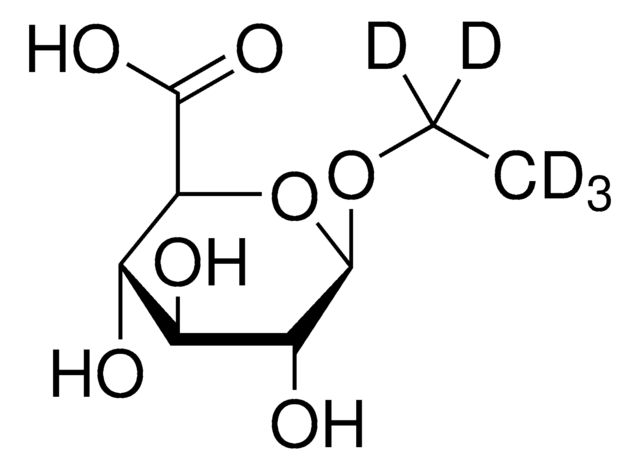
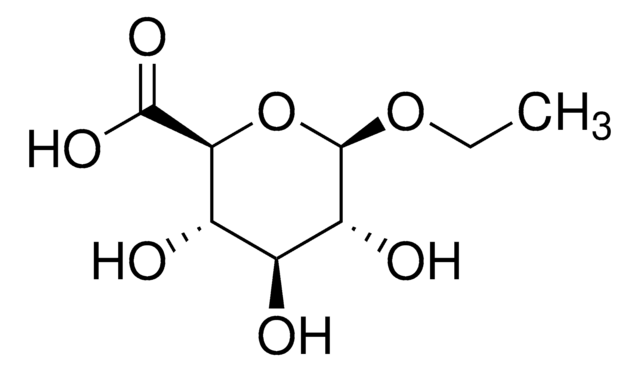
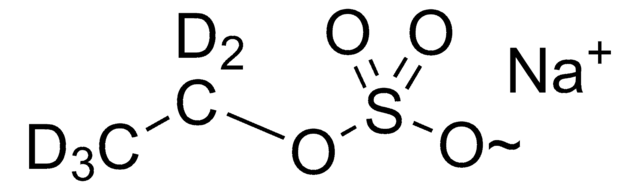
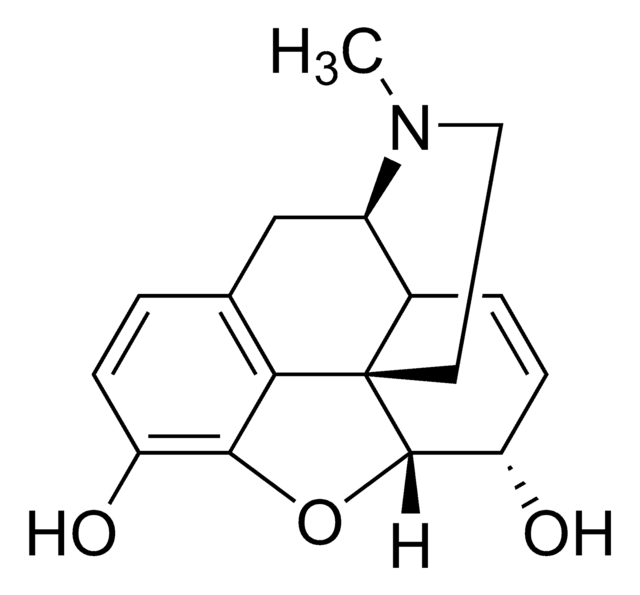
Ethyl-β-D-glucuronide
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Recommended Products
grade
certified reference material
form
liquid
feature
Snap-N-Spike®/Snap-N-Shoot®
packaging
ampule of 1 mL
manufacturer/tradename
Cerilliant®
concentration
1.0 mg/mL in methanol
technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
application(s)
forensics and toxicology
format
single component solution
storage temp.
−20°C
SMILES string
O[C@H]1[C@H](O)[C@@H](C(O)=O)O[C@@H](OCC)[C@@H]1O
InChI
1S/C8H14O7/c1-2-14-8-5(11)3(9)4(10)6(15-8)7(12)13/h3-6,8-11H,2H2,1H3,(H,12,13)/t3-,4-,5+,6-,8+/m0/s1
InChI key
IWJBVMJWSPZNJH-UQGZVRACSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Ethyl-β-D-glucuronide (EtG) is a water-soluble and stable metabolite of ethanol, used as a routine biomarker in alcohol abuse monitoring. A very small fraction of ethanol undergoes degradation to EtG, and it can be detected in body fluids as well as hair.
Application
- Multi-residue analysis of different alcohol metabolites in human blood plasma and urine samples by ultra-high performance liquid chromatography (UHPLC) combined with tandem mass spectrometry (MS/MS)
- Optimization of a supercritical fluid chromatography-tandem mass spectrometric (SFC-MS/MS) method for the separation and quantification of highly polar drugs and their metabolites using an ion-exchange stationary phase from human urine samples
- Evaluation of a paired ion electrospray ionization mass spectrometric (PIESI-MS) approach coupled with high performance liquid chromatographic (HPLC) procedure for the detection and measurement of drug metabolites in urine samples
- Assess the impact of sample preparation of hair samples on the quantitative analysis of ethyl-β-D-glucuronide by ultra-high performance liquid chromatography-electrospray ionization-tandem mass spectrometry
- Analysis of ethyl-β-D-glucuronide as a urinary biomarker in human urine samples by a benchtop gas chromatograph coupled with a mass spectrometer (GC-MS)
Features and Benefits
- Fully characterized under ISO/IEC 17025 and ISO 17034 accreditation
- Accompanied with a comprehensive Certificate of Analysis (CoA) with data on stability, homogeneity, accuracy of concentration, uncertainty, and traceability
- Rigorously tested through real-time stability studies to ensure accuracy and shelf life
- Gravimetrically prepared using qualified precision balances to ensure minimal uncertainty
- Flame sealed under argon into ampoules for long-term shelf life
- Offered in a convenient, DEA-exempt format to improve laboratory efficiency
Legal Information
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
Target Organs
Eyes
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
49.5 °F - closed cup
Flash Point(C)
9.7 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Review of clinically relevant ethanol metabolites and analytical method development for the analysis of ethyl glucuronide and ethyl sulfate in urine matrix.
The method shown uses HILIC mode on an Ascentis® Express OH5 column to retain both analytes well, making it very likely to be more robust and reliable, as well as highly MS-friendly.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service