900127E
Avanti
14(15) EET
Avanti Research™ - A Croda Brand, ethanol solution
Synonym(s):
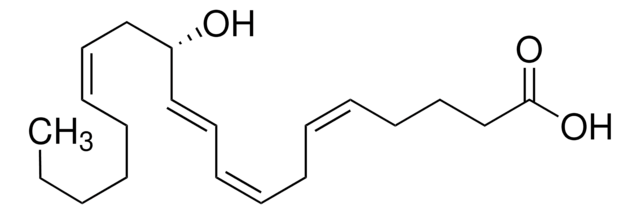
(±)14(15)-epoxy-5Z,8Z,11Z-eicosatrienoic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C20H32O3
CAS Number:
Molecular Weight:
320.47
UNSPSC Code:
12352211
NACRES:
NA.25
Recommended Products
Assay
99% (TLC)
form
ethanol solution
packaging
pkg of 1 × 500 μg (900127E-500UG)
manufacturer/tradename
Avanti Research™ - A Croda Brand
concentration
500 μg/mL (900127E-500UG)
lipid type
neutral glycerides
shipped in
dry ice
storage temp.
−20°C
General description
Epoxytrienoic acid (EET) is produced from arachidonic acid. 14(15) EET is an EET, which is produced by five cytochrome P450 (CYP) epoxygenases. It is a regioisomer of EET synthesized in mammalian tissues.
Biochem/physiol Actions
Epoxytrienoic acid (EET) plays a role as lipid mediator in the renal and cardiovascular systems. 14(15) EET activates many signal transduction pathways by binding to the membrane receptors. It possesses vasoactive properties.
Packaging
2ML Amber Glass Sealed Ampule (900127E-500UG)
Legal Information
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
55.4 °F
Flash Point(C)
13 °C
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Cytochrome P450 epoxygenase pathway of polyunsaturated fatty acid metabolism
Spector A A and Kim H Y
Biochimica et Biophysica Acta - Molecular and Cell Biology of Lipids, 1851(4), 356-365 (2015)
Cytochrome P450 and arachidonic acid bioactivation: molecular and functional properties of the arachidonate monooxygenase
Capdevila J H, et al.
Journal of Lipid Research, 41(2), 163-181 (2000)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service