W241811
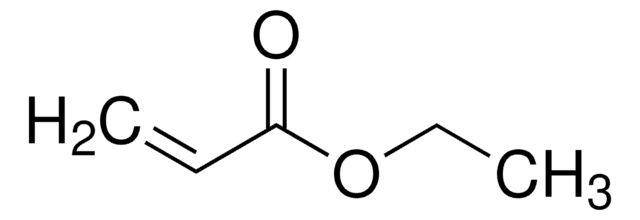
Ethyl acrylate
≥99.5%, stabilized
Synonym(s):
Acrylic acid ethyl ester, Ethyl 2-propenoate
About This Item
Recommended Products
biological source
synthetic
Quality Level
grade
Kosher
reg. compliance
FDA 21 CFR 117
vapor density
3.5 (vs air)
vapor pressure
31 mmHg ( 20 °C)
Assay
≥99.5%
form
liquid
autoignition temp.
721 °F
contains
10-30 ppm MEHQ as stabilizer (synthetic)
expl. lim.
12.1 %
refractive index
n20/D 1.406 (lit.)
bp
99 °C (lit.)
mp
−71 °C (lit.)
density
0.918 g/mL at 25 °C (lit.)
application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
food allergen
no known allergens
Organoleptic
plastic
SMILES string
CCOC(=O)C=C
InChI
1S/C5H8O2/c1-3-5(6)7-4-2/h3H,1,4H2,2H3
InChI key
JIGUQPWFLRLWPJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Refractive index adjustable intraocular lens design to achieve diopter control for improving the treatment of ametropia after cataract surgery.: This study involves the use of ethyl acrylate in developing intraocular lenses with adjustable refractive indices, which aims to improve visual outcomes in patients undergoing cataract surgery by providing customizable diopter control (Hong et al., 2024).
Disclaimer
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
48.2 °F - closed cup
Flash Point(C)
9 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service