K0875
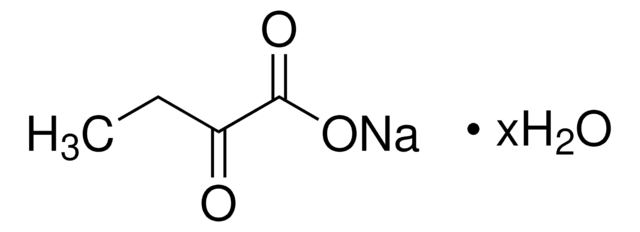
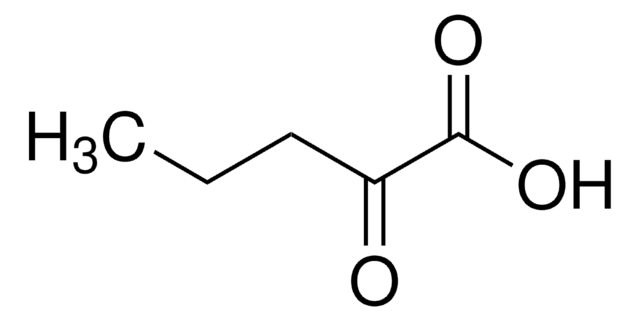
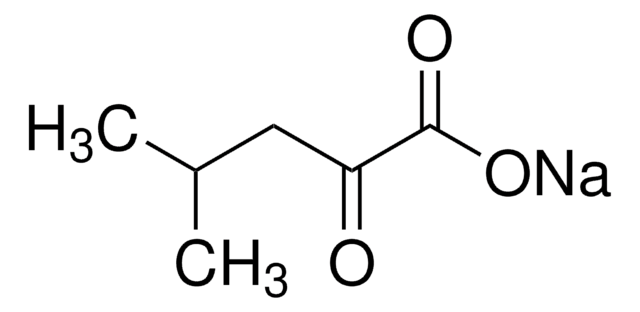
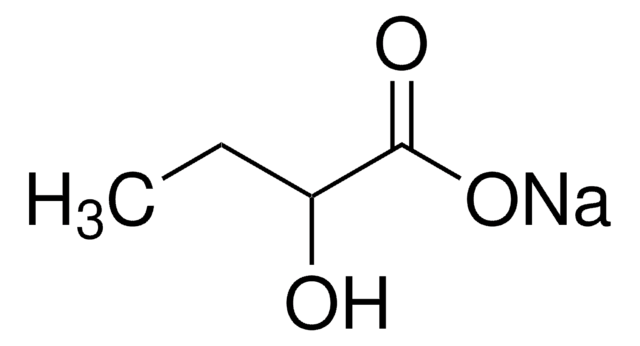
Sodium 2-oxobutyrate
powder
Synonym(s):
2-Oxobutanoic acid sodium salt, 2-Oxobutyric acid sodium salt, Sodium α-ketobutyrate
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
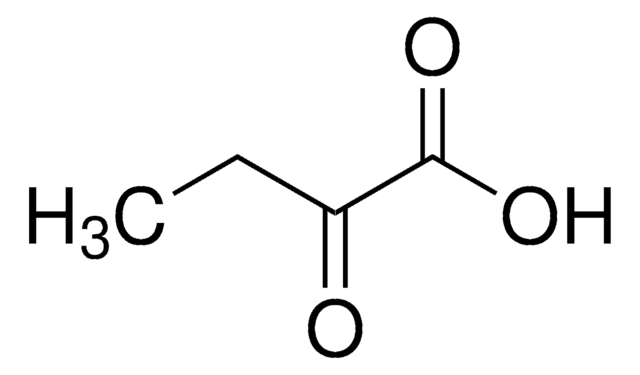
CH3CH2COCOONa
CAS Number:
Molecular Weight:
124.07
Beilstein:
3631701
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
powder
Quality Level
color
white
storage temp.
2-8°C
SMILES string
[Na+].CCC(=O)C([O-])=O
InChI
1S/C4H6O3.Na/c1-2-3(5)4(6)7;/h2H2,1H3,(H,6,7);/q;+1/p-1
InChI key
SUAMAHKUSIHRMR-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Sodium 2-oxobutyrate can be used in the preparation of metal complexes such as lanthanide poly(imino carboxylate) complexes and half-sandwich complexes of (S)-1-amino-2-(methoxymethyl)-pyrrolidine. It can also be used in the synthesis of antiviral agents, 6-azapyrimidine-2′-deoxy-4′-thionucleosides.
Substrate for the determination of lactate dehydrogenase isoenzymes.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Half-sandwich complexes of (S)-1-amino-2-(methoxymethyl)-pyrrolidine (SAMP).
Hoffmuller W, et al.
Journal of Organometallic Chemistry, 564(1), 179-187 (1998)
Pei-Hsuan Chen et al.
Molecular cell, 76(5), 838-851 (2019-10-01)
Intermediary metabolism in cancer cells is regulated by diverse cell-autonomous processes, including signal transduction and gene expression patterns, arising from specific oncogenotypes and cell lineages. Although it is well established that metabolic reprogramming is a hallmark of cancer, we lack
6-Azapyrimidine-2`-deoxy-4`-thionucleosides: Antiviral Agents against TK+ and TK? HSV and VZV Strains.
Maslen H L, et al.
Journal of Medicinal Chemistry, 47(22), 5482-5491 (2004)
Formation of oligomeric lanthanide complexes with new tripodal poly (imino carboxylate) ligands.
Blake A J, et al.
J. Chem. Soc., Dalton Trans., 20, 3655-3658 (1997)
Sergey V Smirnov et al.
FEMS microbiology letters, 273(1), 70-77 (2007-06-15)
A two-step enzymatic synthesis process of 4-hydroxyisoleucine is suggested. In the first step, the aldol condensation of acetaldehyde and alpha-ketobutyrate catalyzed by specific aldolase results in the formation of 4-hydroxy-3-methyl-2-keto-pentanoate (HMKP). In the second step, amination of HMKP by the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service