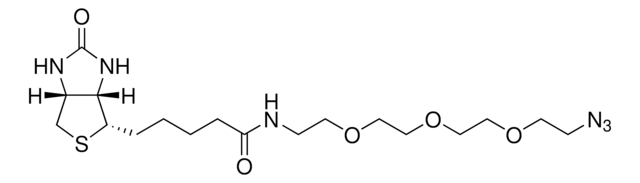
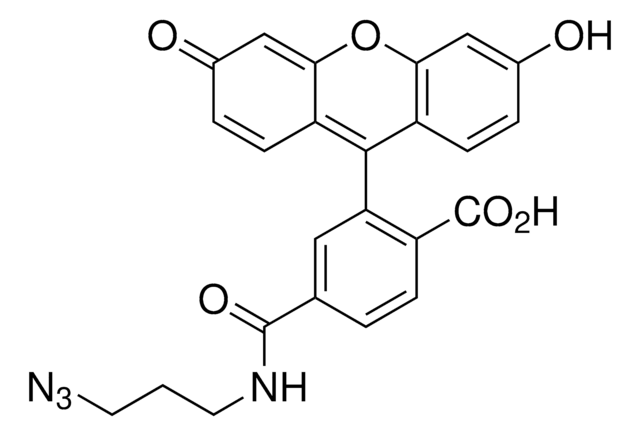
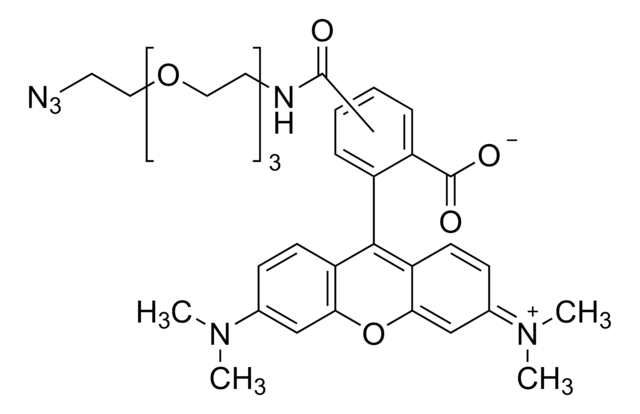
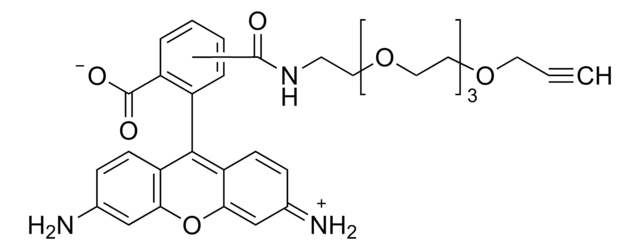
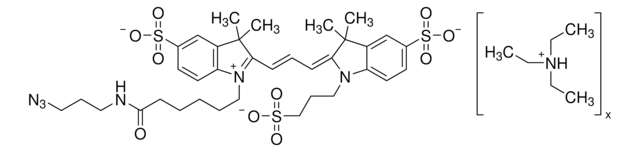
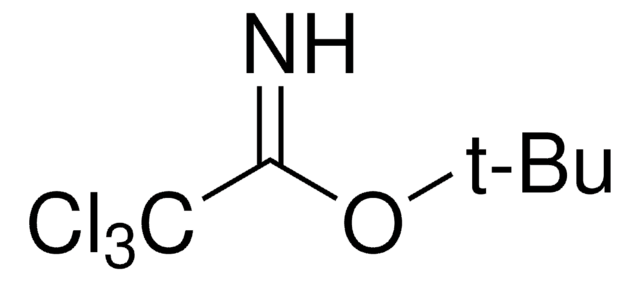
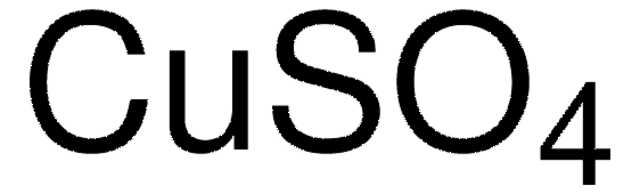906328
BTTAA
≥95%
Synonym(s):
2-(4-((Bis((1-(tert-butyl)-1H-1,2,3-triazol-4-yl)methyl)amino)methyl)-1H-1,2,3-triazol-1-yl)acetic acid, Copper click-chemistry ligand, Water-soluble CuAAC ligand
About This Item
Recommended Products
Assay
≥95%
form
solid
reaction suitability
reaction type: click chemistry
availability
available only in USA
storage temp.
2-8°C
Application
Other Notes
Fast, cell-compatible click chemistry with copper-chelating azides for biomolecular labeling
Metabolic labeling of fucosylated glycoproteins in Bacteroidales species
Increasing the Efficacy of Bioorthogonal Click Reactions for Bioconjugation: A Comparative Study
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Self-react. C
Storage Class Code
5.2 - Organic peroxides and self-reacting hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![Tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine 97%](/deepweb/assets/sigmaaldrich/product/structures/179/695/86a721c8-2a4c-4e4f-bc36-6276ce7a941f/640/86a721c8-2a4c-4e4f-bc36-6276ce7a941f.png)