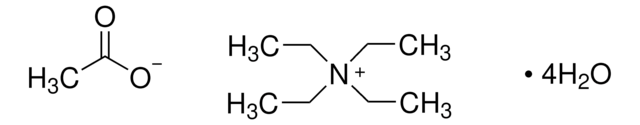
86849
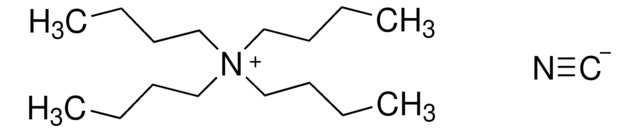
Tetrabutylammonium acetate
technical, ≥90% (T)
Synonym(s):
TBAAc
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3CH2CH2CH2)4N(OCOCH3)
CAS Number:
Molecular Weight:
301.51
Beilstein:
3599376
EC Number:
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical
Quality Level
Assay
≥90% (T)
form
powder
mp
95-98 °C (lit.)
SMILES string
CC([O-])=O.CCCC[N+](CCCC)(CCCC)CCCC
InChI
1S/C16H36N.C2H4O2/c1-5-9-13-17(14-10-6-2,15-11-7-3)16-12-8-4;1-2(3)4/h5-16H2,1-4H3;1H3,(H,3,4)/q+1;/p-1
InChI key
MCZDHTKJGDCTAE-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
General description
Tetrabutylammonium acetate is an effective alternative for sodium acetate (NaOAc) due to its good solubility in organic solvents.
Application
Tetrabutylammonium acetate (TBAAc) is a good source of nucleophilic acetate ion for SN2 substitution reactions. It is commonly used to displace sulfonates and allylic halides to get corresponding acetates. Additionally, TBAAc can also be used as a mild, soluble base in Sonogashira reaction and Heck arylation.
Other Notes
Reagent for the epimerization of hydroxyl groups; base-molten salt for directing Heck-type reactions
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Pd nanoparticle catalyzed Heck arylation of 1, 1-disubstituted alkenes in ionic liquids. Study on factors affecting the regioselectivity of the coupling process.
Calo V, et al.
Organometallics, 22(21), 4193-4197 (2003)
T. Jeffery, M. David
Tetrahedron Letters, 39, 5751-5751 (1998)
Tetrabutylammonium Acetate.
Encyclopedia of Reagents for Organic Synthesis, Second Edition (2001)
Secondary deuterium kinetic isotope effects and the intervention of nonclassical ions in the solvolysis of exo-norborn-2-yl bromobenzene-p-sulfonate.
Maskill H,
Journal of the American Chemical Society, 98(26), 8482-8485 (1976)
G. Battistuzzi et al.
Synlett, 439-439 (2002)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service