704415
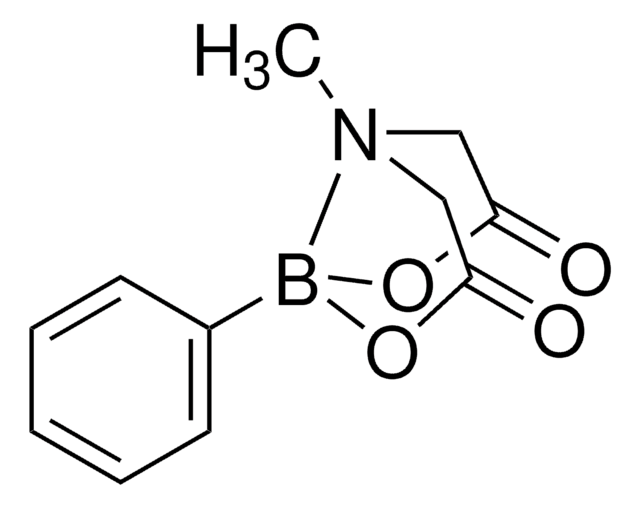
Vinylboronic acid MIDA ester
97%
Synonym(s):
6-Methyl-2-vinyl-1,3,6,2-dioxazaborocane-4,8-dione
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Empirical Formula (Hill Notation):
C7H10BNO4
CAS Number:
Molecular Weight:
182.97
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
powder
mp
152-156 °C
storage temp.
2-8°C
SMILES string
CN1CC(=O)OB(OC(=O)C1)C=C
InChI
1S/C7H10BNO4/c1-3-8-12-6(10)4-9(2)5-7(11)13-8/h3H,1,4-5H2,2H3
InChI key
MGRQGYAVASCCAK-UHFFFAOYSA-N
General description
Vinylboronic acid MIDA ester, like other MIDA boronates, possesses the capacity for controlled, in situ slow-release of boronic acids under aqueous basic conditions allowing the cross-coupling of classically challenging substrates.
Application
MIDA boronates as stable boronic acid surrogates for classically challenging cross-couplings
Suzuki Cross-Coupling with MIDA Boronates
Suzuki Cross-Coupling with MIDA Boronates
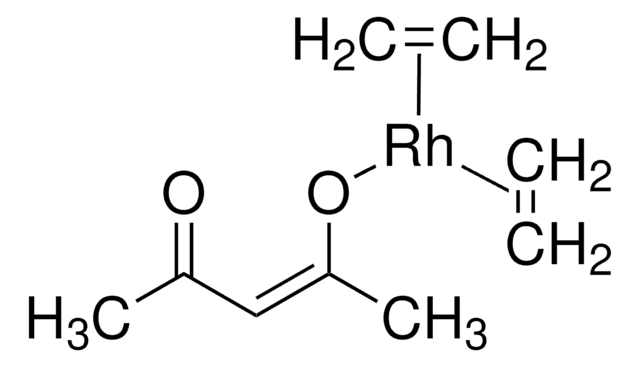
- Vinylboronic acid MIDA ester is an air and chromatographically stable boronic acid surrogate for Suzuki-Miyaura cross-coupling. It can also be used in Heck and oxidative Heck reactions as well as in olefin metathesis to provide the cross-coupled product.
- It is compatible with a wide range of common synthetic reagents that allows functionalization to synthesize structurally complex boronic acid surrogates.
- It undergoes cyclopropanation and epoxidation to yield corresponding MIDA cyclopropane and oxirane, respectively.
- It can be used as one of the major reagents for the scalable synthesis of potent cytotoxin, Leiodermatolide and for the total synthesis of (−)-Blepharocalyxin D.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Vinyl MIDA boronate: a readily accessible and highly versatile building block for small molecule synthesis.
Uno BE, et al.
Tetrahedron, 65(16), 3130-3138 (2009)
Synthesis, molecular editing, and biological assessment of the potent cytotoxin leiodermatolide.
Mailhol D, et al.
Journal of the American Chemical Society, 136(44), 15719-15729 (2014)
Synthesis of trans-2-(Trifluoromethyl) cyclopropanes via Suzuki reactions with an N-methyliminodiacetic acid boronate.
Duncton MA and Singh R.
Organic Letters, 15(17), 4284-4287 (2013)
Total Synthesis of (−)-Blepharocalyxin D and Analogues.
Cons BD, et al.
Organic Letters, 15(8), 2046-2049 (2013)
A general solution for unstable boronic acids: slow-release cross-coupling from air-stable MIDA boronates.
Knapp DM, et al.
Journal of the American Chemical Society, 131(20), 6961-6963 (2009)
Articles
An article regarding MIDA-protected Boronate Esters.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service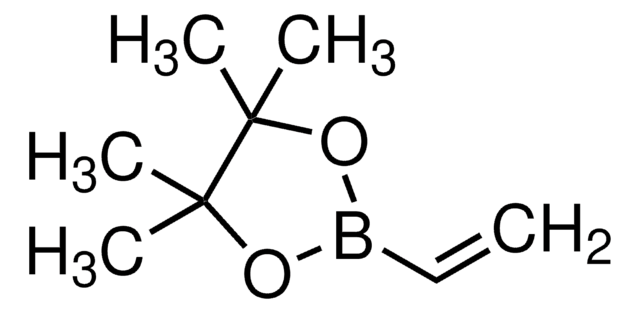
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)