521418
4-Cyanophenylboronic acid
≥95%
Synonym(s):
(p-Cyanophenyl)boronic acid, 4-Cyanobenzeneboronic acid, 4-Cyanophenylboric acid
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Linear Formula:
NCC6H4B(OH)2
CAS Number:
Molecular Weight:
146.94
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95%
mp
>350 °C (lit.)
functional group
nitrile
SMILES string
OB(O)c1ccc(cc1)C#N
InChI
1S/C7H6BNO2/c9-5-6-1-3-7(4-2-6)8(10)11/h1-4,10-11H
InChI key
CEBAHYWORUOILU-UHFFFAOYSA-N
Related Categories
Application
4-Cyanophenylboronic acid can be used as a reactant in:
It can also be used to prepare:
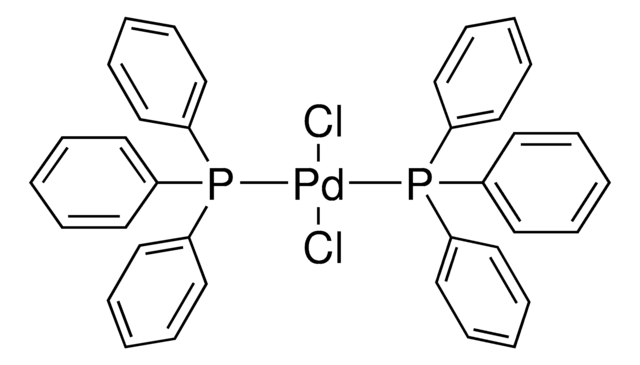
- Palladium-catalyzed Suzuki-Miyaura cross-coupling in water.
- Ruthenium catalyzed direct arylation of benzylic sp3 carbons of acyclic amines with arylboronates.
- Ligand-free copper-catalyzed coupling of nitro arenes with arylboronic acids.
- Ferric perchlorate-promoted reaction of fullerenes with various arylboronic acids to give fullerenyl boronic esters.
- Phosphine-free Suzuki-Miyaura cross-coupling.
- Palladacycles as effective catalysts for multicomponent reaction with allylpalladium-intermediates.
- Chan-Lam-type Cu-catalyzed S-arylation of thiols.
- Regioselective cross-coupling reactions under modfied Suzuki and Still cross-coupling reactions with copper catalysis.
- Metal-free biaryl coupling reaction in the presence of dimethyl carbonate as a solvent.
- Suzuki-type cross-coupling reaction with pentavalent triarylantimony diacetates in the absence of a base.
It can also be used to prepare:
- Himbacine analogs as thrombin receptor antagonists and potential antiplatelet agents.
- Trisulfonated calixarene upper-rim sulfonamido and their complexation with trimethyllysine epigenetic mark.
- Antimalarial compounds via Suzuki cross-coupling.
- Deoxyuridine derivatives.
Reactant involved in:
Precursor in the synthesis of inhibitors such as:
- Oxidative hydroxylation
- Trifluoromethylation
- 1,4-Addition reactions
Precursor in the synthesis of inhibitors such as:
- Tpl2 kinase inhibitors
- P2X7 antagonists used in the treatment of pain
Other Notes
Contains varying amounts of anhydride
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Discovery of nor-seco himbacine analogs as thrombin receptor antagonists
Chelliah, M. V.; et al.
Bioorganic & Medicinal Chemistry, 22, 2544-2549 (2012)
Mohamed A Ismail et al.
Journal of medicinal chemistry, 47(14), 3658-3664 (2004-06-25)
2-[5-(4-Amidinophenyl)-furan-2-yl]-5,6,7,8-tetrahydro-imidazo[1,2-a]pyridine-6-carboxamidine acetate salt (7) was synthesized from 2-[5-(4-cyanophenyl)-furan-2-yl]-imidazo[1,2-a]pyridine-6-carbonitrile (4a), through the bis-O-acetoxyamidoxime followed by hydrogenation in glacial acetic acid. Compound 4a was obtained in four steps starting with two successive brominations of 2-acetylfuran first with N-bromosuccinimide, and second with bromine
A mild and efficient new synthesis of aryl sulfones from boronic acids and sulfinic acid salts
Christian Beaulieu, et al.
Tetrahedron Letters, 45, 3233-3236 (2004)
Palladacycles: Effective catalysts for a multicomponent reaction with allylpalladium(II)-intermediates
Shiota, A.; Malinakova, H. C.
Journal of Organometallic Chemistry, 704, 9-16 (2012)
Yassir Younis et al.
Journal of medicinal chemistry, 55(7), 3479-3487 (2012-03-07)
A novel class of orally active antimalarial 3,5-diaryl-2-aminopyridines has been identified from phenotypic whole cell high-throughput screening of a commercially available SoftFocus kinase library. The compounds were evaluated in vitro for their antiplasmodial activity against K1 (chloroquine and drug-resistant strain)
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 521418-1G | 4061832547312 |
| 521418-10G | 4061832905150 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)