510270
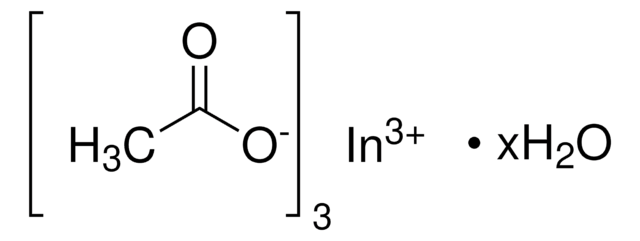
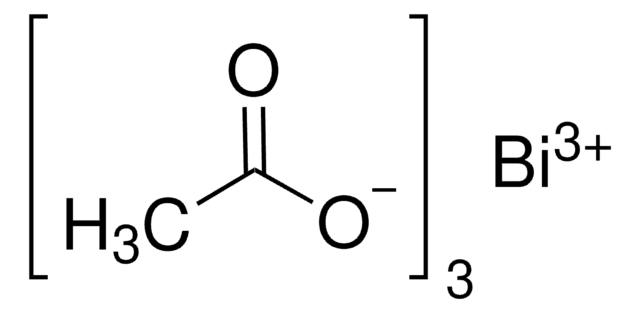
Indium(III) acetate
99.99% trace metals basis
Synonym(s):
Indium triacetate
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
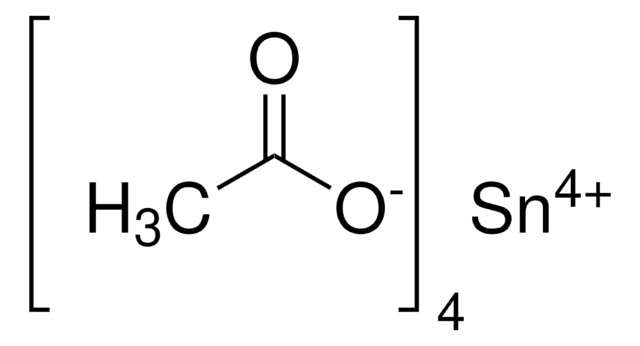
In(C2H3O2)3
CAS Number:
Molecular Weight:
291.95
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
99.99% trace metals basis
form
solid
reaction suitability
core: indium
reagent type: catalyst
mp
270 °C (dec.) (lit.)
SMILES string
CC(=O)O[In](OC(C)=O)OC(C)=O
InChI
1S/3C2H4O2.In/c3*1-2(3)4;/h3*1H3,(H,3,4);/q;;;+3/p-3
InChI key
VBXWCGWXDOBUQZ-UHFFFAOYSA-K
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Indium(III) acetate is a white crystalline water-soluble solid that decomposes to indium oxide upon heating. It is used as a catalyst in intermolecular and intramolecular acyl substitution reactions. It is also widely used as CVD precursor to prepare indium oxide thin oxide.
Application
Indium(III) acetate can be used:
- As a precursor to fabricate In2S3 thin films via chemical spray pyrolysis. These films are used as electron transport layers in highly efficient perovskite solar cells.
- To prepare indium arsenide quantum dots which are infrared emitting nanomaterials used in optoelectronic and biomedical applications.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kinetics analysis for non-isothermal decomposition ?-irradiated indium acetate.
Al-Resayes SI, et al
Arabian Journal of Chemistry, 3(3), 191-194 (2010)
Characteristics of CuInS2/ZnS quantum dots and its application on LED
Kim H,et al
Journal of Crystal Growth, 326, 90-93 (2011)
Katsukiyo Miura et al.
Organic letters, 10(1), 133-136 (2007-12-13)
In the presence of phenylsilane and a catalytic amount of indium(III) acetate, organic iodides added to electron-deficient alkenes in ethanol at room temperature. Both simple and functionalized organic iodides were applicable to this reaction. A plausible reaction mechanism involves the
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 510270-10G | 4061832490168 |
| 510270-50G | 4061832490182 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service